2,5-二氯苯酰肼 | 67487-35-8
中文名称
2,5-二氯苯酰肼
中文别名
2,5-二氯苯甲酰肼
英文名称
2,5-dichlorobenzhydrazide
英文别名
2,5-dichlorobenzoic hydrazide;2,5-dichlorobenzohydrazide
CAS
67487-35-8
化学式
C7H6Cl2N2O
mdl
MFCD00014754
分子量
205.043
InChiKey
XECBCXNWGBDIMG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:178-182 °C
-
密度:1.455±0.06 g/cm3(Predicted)
-
稳定性/保质期:
远离氧化物。
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:12
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:55.1
-
氢给体数:2
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
海关编码:2928000090
-
安全说明:S26,S37/39
-
危险性防范说明:P261,P264,P270,P271,P280,P301+P312,P302+P352,P304+P340,P305+P351+P338,P330,P332+P313,P337+P313,P362,P403+P233,P405,P501
-
危险性描述:H302,H312,H315,H319,H332,H335
-
储存条件:存放在密封容器中,并置于阴凉、干燥处。务必远离氧化剂。
SDS
| Name: | 2 5-Dichlorobenzoic hydrazide 98% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 67487-35-8 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 67487-35-8 | 2,5-Dichlorobenzoic hydrazide | 98% | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 67487-35-8: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: off-white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 178 - 182 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: Insoluble.
Specific Gravity/Density:
Molecular Formula: C7H6Cl2N2O
Molecular Weight: 205.04
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 67487-35-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,5-Dichlorobenzoic hydrazide - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 67487-35-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 67487-35-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 67487-35-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:参考文献:名称:3-(1-substituted-4-piperazinyl)-1H-indazoles摘要:本发明涉及具有以下通式结构的3-(1-取代-4-哌嗪基)-1H-吲唑类化合物:##STR1## 其中R1为氢、低级烷基、芳基低级烷基、酰基、环烷基低级亚烷基和苯磺酰基;R2为氢、低级烷基、羟基低级烷基,其结构式为##STR2## 其中Z选自氢、卤素、低级烷氧基、CF3、NO2和NH2;##STR3## 其中Z如上定义;##STR4## 其中Z如上定义;##STR5## 其中R3和R4各自独立为氢和低级烷基;##STR6## 其中Z如上定义;##STR7## 其中R'和R"各自独立为氢和低级烷基;X为氢、低级烷基、羟基、卤素、低级烷氧基、CF3、NO2和氨基;n为1至4的整数,但当R1为氢或酰基且X为氯时,R2不应当为低级烷基;其药学上可接受的酸加成盐,以及适当时的几何异构体、立体异构体和外消旋混合物。本发明的化合物显示出作为镇痛剂和抗精神病药物的效用。公开号:US04954503A1
-
作为产物:描述:参考文献:名称:含1,3,4-恶二唑环的(E)-α-(甲氧基亚氨基)-苯乙酸酯衍生物的立体选择性合成和杀真菌活性。摘要:立体选择性地合成了十五种新颖的(E)-α-(甲氧基亚氨基)-苯乙酸酯衍生物,它们是嗜球果伞素的类似物,其包含(E)-甲氧基亚氨基乙酸甲酯部分和1,3,4-恶二唑环的两个药效学亚结构。首先发现偶联反应可以以14:1的比例立体选择性地产生关键中间体(E)和2-(羟基亚氨基)-2-邻甲苯基乙酸酯(Z)-甲基。初步的生物测定表明,所有化合物1对茄根霉,灰葡萄孢,玉米赤霉菌,皮氏假单胞菌和双极性芽孢杆菌均显示出有效的杀真菌活性,并且所有测试化合物1a-1o均比Kresoxim-具有更强的对茄霉的杀真菌活性。甲基。DOI:10.1016/j.bmcl.2006.01.026
文献信息
-
Synthesis, Characterization and Antimicrobial Evaluation of (E)-N'-[(1-(2-methoxy-6-pentadecylbenzyl)-1H-1,2,3-triazol-4-yl]- methylene)benzohydrazide Derivatives作者:N. Rambabu、P.K. Dubey、B. Ram、B. BalramDOI:10.14233/ajchem.2016.19310日期:——Anacardic acid (pentadecyl salicylic acid) is a phenolic constituent present in cashew nut shell liquid (Anacardium occidentale L.) and exhibits antimicrobial properties. The present paper describes the synthesis, characterization and antimicrobial evaluation of hydrazone derivatives of anacardic acid (9a-l) linked with 1,2,3-triazole ring. All the newly synthesized compounds were determined by 1H NMR, mass and IR spectroscopy. Compounds 9d, 9e, 9h, 9i and 9j exhibited strong antifungal activity against the tested fungal strains viz., A. niger and C. albicans.
-
Hydrazide and alkoxyamide angiogenesis inhibitors申请人:——公开号:US20020002152A1公开(公告)日:2002-01-03Compounds having the formula 1 are methionine aminopeptidase type 2 (MetAP2) inhibitors and are useful for inhibiting angiogenesis. Also disclosed are MetAP2-inhibiting compositions and methods of inhibiting angiogenesis in a mammal.
-
Synthesis and Antibacterial Activity of (E)-N'-[4-{2-(4-Fluorophenylthio)ethoxy}-3-cyano-5-methoxybenzylidene]-substituted benzohydrazide Derivatives作者:Lokamaheshwari Dommati、B. Satyanarayana、P. Gayatri Hela、B. Ram、G. SrinivasDOI:10.14233/ajchem.2016.19776日期:——Commercially available vanillin was used as the starting material for the preparation of some new (E)-N’-[4-2-(4-fluorophenylthio)-ethoxy}-3-cyano-5-methoxybenzylidene]-4-substituted benzohydrazide derivatives (8.1 to 8.10) in quantitative yields. The structural confirmations of all the newly synthesized hydrazone derivatives were established on the basis of 1H NMR, mass and IR data. Hydrazides such as (E)-N’-[4-2-(4-fluorophenylthio)ethoxy}-3-cyano-5-methoxybenzylidene]-2,5-dichlorobenzohydrazide (8.6), (E)-N’-[4-2-(4-fluorophenylthio)ethoxy}-3-cyano-5-methoxybenzylidene]-2,5-difluorobenzohydrazide (8.9) showed duplication of NMR signals, this was attributed to the presence of anti and syn periplanar conformers. Antibacterial study against Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa with reference to the standard drug (streptomycin) revealed that compounds bearing R = 4-OH (8.2), 3,4,5-OMe (8.3) and 4-SO2Me (8.4) substituents has shown the good antibacterial sensitivity.以市售的香草醛为起始原料,制备了一系列新型的(E)-N’-[4-2-(4-氟苯硫基)乙氧基}-3-氰基-5-甲氧基亚苄基]-4-取代苯甲酰肼衍生物(8.1至8.10),产率为定量。所有新合成的脎衍生物的结构确认基于1H NMR、质谱和红外数据。例如(E)-N’-[4-2-(4-氟苯硫基)乙氧基}-3-氰基-5-甲氧基亚苄基]-2,5-二氯苯甲酰肼(8.6)和(E)-N’-[4-2-(4-氟苯硫基)乙氧基}-3-氰基-5-甲氧基亚苄基]-2,5-二氟苯甲酰肼(8.9)显示出NMR信号的重复,这归因于反式和顺式periplanar构象的存在。针对金黄色葡萄球菌、枯草芽孢杆菌、大肠杆菌和铜绿假单胞菌的抗菌研究,参考标准药物(链霉素),结果显示含有R=4-OH(8.2)、3,4,5-OMe(8.3)和4-SO2Me(8.4)取代基的化合物表现出良好的抗菌敏感性。
-
[EN] 1-ARYL-4-METHYL-[1,2,4]TRIAZOLO[4,3-a]QUINOXALINE DERIVATIVES<br/>[FR] DÉRIVÉS DE 1-ARYL-4-MÉTHYL-[1,2,4]TRIAZOLO[4,3-A]QUINOXALINE申请人:JANSSEN PHARMACEUTICA NV公开号:WO2013000924A1公开(公告)日:2013-01-03The present invention relates to novel l-aryl-4-methyl-[l,2,4]triazolo[4,3-a]- quinoxaline derivatives as inhibitors of phosphodiesterase 2 (PDE2) and to a lesser extent of phosphodiesterase 10 (PDE10) or as inhibitors of both, phosphodiesterases 2 and 10. The invention is also directed to pharmaceutical compositions comprising such compounds, to processes for preparing such compounds and compositions, and to the use of such compounds and compositions for the prevention and treatment of disorders in which PDE2 is involved, or disorders in which both PDE2 and PDE10 are involved, such as neurological and psychiatric disorders, and endocrinological or metabolic diseases. The present invention also relates to radiolabeled compounds which may be useful for imaging and quantifying the PDE2 enzyme in tissues, using positron- emission tomography (PET). The invention is also directed to compositions comprising such compounds, to processes for preparing such compounds and compositions, to the use of such compounds and compositions for imaging a tissue, cells or a host, in vitro or in vivo and to precursors of said compounds.本发明涉及新型的l-芳基-4-甲基-[1,2,4]三唑并[4,3-a]-喹喔啉衍生物,作为磷酸二酯酶2(PDE2)的抑制剂,以及在较小程度上作为磷酸二酯酶10(PDE10)的抑制剂或作为磷酸二酯酶2和10的双重抑制剂。该发明还涉及包含这类化合物的药物组合物,用于制备这类化合物和组合物的方法,以及将这类化合物和组合物用于预防和治疗涉及PDE2的疾病或涉及PDE2和PDE10的疾病,如神经和精神疾病,内分泌或代谢性疾病。本发明还涉及可能用于组织中PDE2酶的成像和定量的放射标记化合物,使用正电子发射断层扫描(PET)。该发明还涉及包含这类化合物的组合物,用于制备这类化合物和组合物的方法,将这类化合物和组合物用于体内或体外成像组织、细胞或宿主,并涉及这类化合物的前体。
-
[EN] AMINO-QUINOXALINE AND AMINO-QUINOLINE COMPOUNDS FOR USE AS ADENOSINE A2a RECEPTOR ANTAGONISTS<br/>[FR] AMINO-QUINOXALINE ET COMPOSÉS AMINO-QUINOLINE À UTILISER EN TANT QU'ANTAGONISTES DU RÉCEPTEUR A2a申请人:SCHERING CORP公开号:WO2009111442A1公开(公告)日:2009-09-11Compounds of the Formula (I), where W represents CH or N; and Q represents -CN, -C(=NOH)NH2, -CONHR1 or various herein described heterocyclic radicals; as well as pharmaceutically acceptable salts, solvates, esters and prodrugs thereof are adenosine A2a receptor antagonists and, therefore, are useful in the treatment of central nervous system diseases, in particular Parkinson's disease.
表征谱图
-
氢谱1HNMR
-
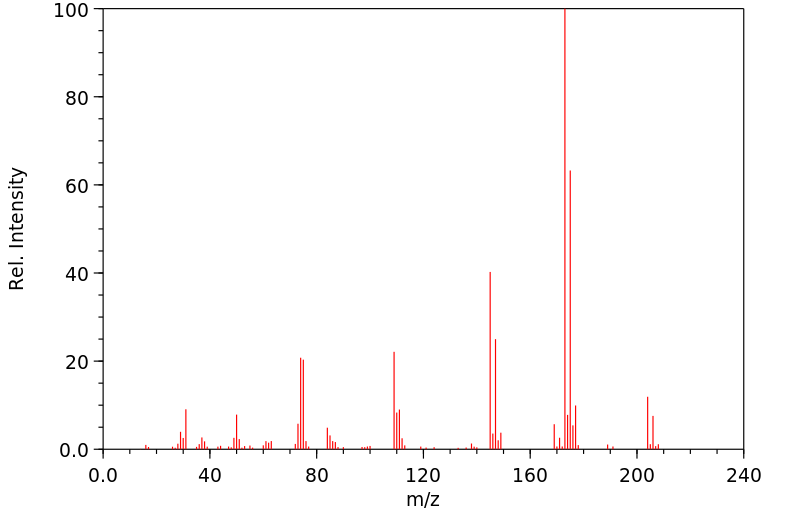
质谱MS
-
碳谱13CNMR
-
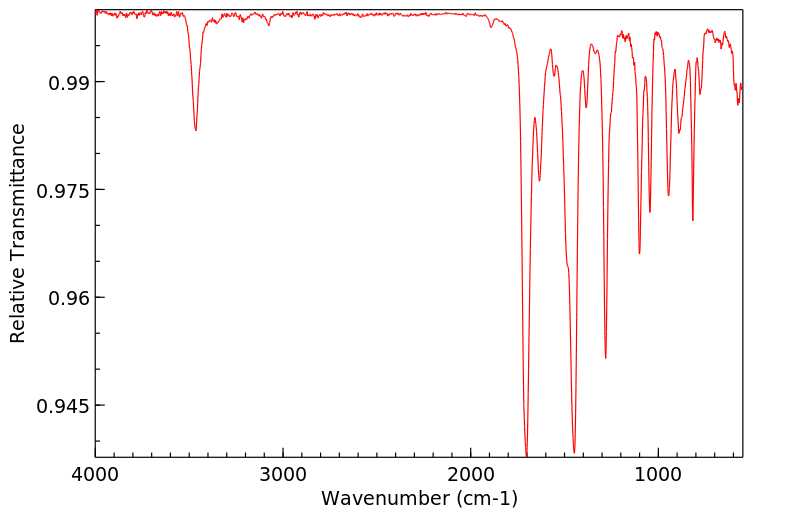
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫