2-(2-萘氧基)乙醇 | 93-20-9
中文名称
2-(2-萘氧基)乙醇
中文别名
2-(2-羟基氧基)萘
英文名称
2-(2-naphthalenyloxy)ethanol
英文别名
2-(naphthalen-2-yloxy)ethanol;2-(2-naphthoxy)ethanol;2-(2-naphthyloxy)ethanol;2-(naphthalene-2-yloxy)ethanol;2-(naphthalen-2-yloxy)ethan-1-ol;2-naphthalen-2-yloxyethanol
CAS
93-20-9
化学式
C12H12O2
mdl
MFCD00016809
分子量
188.226
InChiKey
BQPBZDSDFCDSAO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:74-76°C
-
沸点:355.2±15.0 °C(Predicted)
-
密度:1.164±0.06 g/cm3(Predicted)
-
LogP:1.44 at 20℃
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:14
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:29.5
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xn,F,Xi
-
WGK Germany:3
-
海关编码:2909499000
-
危险类别:3
-
安全说明:S16,S26,S36,S37/39
-
危险类别码:R20/21/22,R36/37/38,R10,R36/37,R11
-
包装等级:III
-
危险品运输编号:UN 1993 3/PG 2
-
储存条件:室温
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-萘氧乙酸 (2-naphthoyl)oxyacetic acid 120-23-0 C12H10O3 202.21 —— di-2-naphthoxymethane 33257-36-2 C21H16O2 300.357 2-萘酚 β-naphthol 135-19-3 C10H8O 144.173 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-[2]naphthyloxy-2-vinyloxy-ethane 26576-19-2 C14H14O2 214.264 [2]萘氧基乙醛 [2]naphthyloxy-acetaldehyde 106328-95-4 C12H10O2 186.21 —— 2-(2-chloroethoxy)naphthalene 3383-79-7 C12H11ClO 206.672 2-(2-萘氧基)乙胺 2-(2-aminoethoxy)naphthalene 23314-24-1 C12H13NO 187.241 —— 2-(naphthalen-2-yloxy)ethyl acetate 6807-14-3 C14H14O3 230.263 —— propan-2-one O-2-(naphthalen-2-yloxy)ethyloxime 1233087-37-0 C15H17NO2 243.305 2-丙烯酸,2-(2-萘基OXY)乙基酯 2-(2-Naphthyloxy)ethyl acrylate 64022-15-7 C15H14O3 242.27 —— 2-(2-mesyloxyethoxy)naphthalene 63649-88-7 C13H14O4S 266.318 —— sulfuric acid mono-(2-[2]naphthyloxy-ethyl ester) —— C12H12O5S 268.29 —— 2-[[2-(2-naphthalenyloxy)ethyl]oxy]thiophene —— C16H14O2S 270.352 —— N-(2-(naphthalen-2-yloxy)ethyl)ethenesulfonamide —— C14H15NO3S 277.344 —— 2-(2-(naphthalen-2-yloxy)ethyl)cyclopentan-1-one —— C17H18O2 254.329 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Antileishmanial Ring-Substituted Ether Phospholipids摘要:Three series of ring-substituted ether phospholipids were synthesized carrying N,N,N-trimethylammonium, N-methylpiperidino, or N-methylmorpholino headgroups. The first series is substituted by 2-cyclohexyloxyethyl or 2-(4-alkylidenecyclohexyloxy)ethyl groups, the second series by cyclohexylidenealkyl or adamantylidenealkyl moieties, and the third series by 2-aryloxyethyl or 6-aryloxyhexyl groups in the alkyl portion of the molecule. The antileishmanial activity of the new compounds was evaluated in vitro against the promastigote forms of L. donovani and L. infantum using an MTT (3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide)-based microassay as a marker of cell viability. Analogues 12, 15, 24, 30, 32, 41, 43, and 45 were more potent than the control compound miltefosine (hexadecylphosphocholine) against both L. donovani and L. infantum while, derivatives 13 and 42 were equipotent to miltefosine. Analogues 16, 17, 19, 20 were more potent than miltefosine against L. infantum and compounds 27, 31, 44 were more active than miltefosine against L. donovani. Differential scanning calorimetry (DSC) was used to probe the role of individual ether phospholipids on the physicochemical properties of model membranes. The DSC scans showed that the active compounds have a more profound effect on the thermotropic properties of model membrane bilayers than the less active ones.DOI:10.1021/jm020972c
-
作为产物:参考文献:名称:TiO2负载的Re作为将羧酸加氢为醇的通用和化学选择性多相催化剂摘要:发现TiO 2负载的Re,Re / TiO 2可以促进具有芳香族和脂肪族部分的羧酸选择性氢化为相应的醇。与其他过渡金属负载的TiO 2和负载的Re催化剂相比,Re / TiO 2具有更好的效果,可用于3-苯基丙酸的选择性加氢。在温和的条件下(140℃下5 MPa H 2)以97%的产率生产3-苯丙醇。与典型的非均相催化剂相反,Re / TiO 2不会导致脱芳构化副产物的形成。该催化剂是可回收的,并且在醇的合成中显示出广泛的底物范围(22个实例;分离产率高达97%)。DOI:10.1002/chem.201604762
文献信息
-
Method of treating lipidemia with aryloxyalkylaminobenzoic acids and
-
[EN] (THIO)MORPHOLINE DERIVATIVES AS S1P MODULATORS<br/>[FR] DÉRIVÉS DE (THIO)MORPHOLINE MODULATEURS DE S1P申请人:ABBOTT HEALTHCARE PRODUCTS BV公开号:WO2011023795A1公开(公告)日:2011-03-03The present invention relates to (thio)morpholine derivatives of the formula (I), wherein R1 is selected from cyano, (2-4C)alkynyl, (1-4C)alkyl, (3-6C)cycloalkyl, (4-6C)cycloalkenyl, (6-8C)bicycloalkyl, (8-10C)bicyclic group, each optionally substituted with (1-4C)alkyl, phenyl, biphenyl, naphthyl, each optionally substituted with one or more substituents independently selected from halogen, (1-4C)alkyloptionally substituted with one or more fluoro atoms, (2-4C)alkynyl, (1-4C)alkoxy optionally substituted with one or more fluoro atoms,amino, di(1-4C)alkylamino, -SO2-(1-4C)alkyl, -CO-(1-4C)alkyl, -CO-O-(1-4C)alkyl, -NH-CO-(1-4C)alkyl and (3-6C)cycloalkyl, phenyl substituted with phenoxy, benzyl, benzyloxy, phenylethyl or monocyclic heterocycle, each optionally substituted with (1-4C)alkyl, monocyclic heterocycle optionally substituted with halogen, (1-4C)alkyl or with phenyl optionally substituted with (1-4C)alkyl, and bicyclic heterocycle optionally substituted with (1-4C)alkyl; A is selected from -CO-O-, -O-CO-, -NH-CO-, -CO-NH, -C=C-, -CCH3-O- and the linking group –Y-(CH2)n-X- wherein Y is attached to R1 and selected from a bond, -O-, -S-, -SO-, -SO2-, -CH2-O-, -CO-, -O-CO-, -CO-O-, -CO-NH-, -NH-CO-, -C=C-and -C≡C-; n is an integer from 1 to 10; and X is attached to the phenylene / pyridyl group and selected from a bond, -O-, -S-, -SO-, -SO2 -, -NH, -CO-, -C=C-and -C≡C-; ring structure B optionally contains one nitrogen atom; R2 is H, (1-4C)alkyl optionally substituted with one or more fluoro atoms, (1-4C)alkoxy optionally substituted with one or more fluoro atoms, or halogen; and R3 is (1-4C)alkylene-R5 wherein the alkylene group may be substituted with (CH2)2 to form a cyclopropyl moiety or one or two halogen atoms, or R3 is is (3-6C)cycloalkylene-R5 or -CO-CH2-R5, wherein R5 is -OH, -PO3H2, -OPO3H2, -COOH, -COO(1-4C)alkyl or tetrazol-5-yl; R4 is H or (1-4C)alkyl; R6 is one or more substituents independently selected from H, (1-4C)alkyl or oxo; W is -O-, -S-, -SO- or -SO2-; or a pharmaceutically acceptable salt, a solvate or hydrate thereof; with the proviso that the derivative of formula (I) is not 2-(4-ethylphenyl)-4-morpholinoethanol or 4-[4-(2-hydroxyethyl)-2-morpholinyl]benzeneacetonitrile or a pharmaceutically acceptable salt, a solvate or hydrate thereof. The compounds of the invention have affinity to S1P receptors and may be used in the treatment, alleviation or prevention of S1P receptor mediated diseases and conditions.本发明涉及公式(I)的(硫)吗啉衍生物,其中R1从氰基,(2-4C)炔基,(1-4C)烷基,(3-6C)环烷基,(4-6C)环烯基,(6-8C)双环烷基,(8-10C)双环基团中选择,每个基团可选择地取代为(1-4C)烷基,苯基,联苯基,萘基,每个基团可选择地取代为一个或多个取代基,独立选择自卤素,(1-4C)烷基可选择地取代为一个或多个氟原子,(2-4C)炔基,(1-4C)氧烷基可选择地取代为一个或多个氟原子,氨基,二(1-4C)烷基氨基,-SO2-(1-4C)烷基,-CO-(1-4C)烷基,-CO-O-(1-4C)烷基,-NH-CO-(1-4C)烷基和(3-6C)环烷基,苯基取代为苯氧基,苄基,苄氧基,苯乙基或单环杂环烃,每个基团可选择地取代为(1-4C)烷基,单环杂环烃可选择地取代为卤素,(1-4C)烷基或取代为苯基的苯基,可选择地取代为(1-4C)烷基,和双环杂环烃可选择地取代为(1-4C)烷基;A从-CO-O-,-O-CO-,-NH-CO-,-CO-NH,-C=C-,-CCH3-O-和连接基-Y-(CH2)n-X-中选择,其中Y连接到R1并从键,-O-,-S-,-SO-,-SO2-,- -O-,-CO-,-O-CO-,-CO-O-,-CO-NH-,-NH-CO-,-C=C-和-C≡C-中选择;n是1到10的整数;X连接到苯基/吡啶基团并从键,-O-,-S-,-SO-,-SO2-,-NH,-CO-,-C=C-和-C≡C-中选择;环结构B可选择地含有一个氮原子;R2是H,(1-4C)烷基可选择地取代为一个或多个氟原子,(1-4C)氧烷基可选择地取代为一个或多个氟原子,或卤素;R3是(1-4C)烷基-R5,其中烷基基团可取代为( )2形成环丙基基团或一个或两个卤素原子,或R3是(3-6C)环烷基-R5或-CO- -R5,其中R5是-OH,-PO3H2,-OPO3H2,-COOH,-COO(1-4C)烷基或四唑-5-基;R4是H或(1-4C)烷基;R6是一个或多个取代基,独立选择自H,(1-4C)烷基或氧代基;W是-O-,-S-,-SO-或-SO2-;或其药学上可接受的盐,溶剂或水合物;但是,公式(I)的衍生物不是2-(4-乙基苯基)-4-吗啉乙醇或4-[4-(2-羟乙基)-2-吗啉基]苯乙腈或其药学上可接受的盐,溶剂或水合物。本发明的化合物具有对S1P受体的亲和力,可用于治疗、缓解或预防S1P受体介导的疾病和症状。
-
GLUTATHIONE-CHOLESTEROL DERIVATIVES AS BRAIN TARGETING AGENTS申请人:South Dakota Board of Regents公开号:US20200048305A1公开(公告)日:2020-02-13The present invention describes compositions containing cholesterol-linker-glutathione conjugates for targeting the brain by overcoming barrier entry to the CNS through the blood brain barrier (BBB), including micelle and liposome forms of such compositions. In addition, methods for treating subjects by administering such compositions are also disclosed.
-
Omega-hydrofluoroalkyl ethers, precursor carboxylic acids and derivatives thereof, and their preparation and application申请人:MINNESOTA MINING AND MANUFACTURING COMPANY公开号:EP1170275A3公开(公告)日:2004-04-14Normally liquid, omega-hydrofluoroalkyl ether compounds (and selected mixtures thereof) have a saturated perfluoroaliphatic chain of carbon atoms interrupted by one or more ether oxygen atoms. The compounds can be prepare, e.g. by decarboxylation of the corresponding fluoroalkyl ether carboxylic acids and are useful, e.g., in cleaning and drying applications.
-
Naphthyloxyalkylaminobenzoic acids, salts and esters thereof申请人:American Cyanamid Company公开号:US04260816A1公开(公告)日:1981-04-07This disclosure describes pharmaceutical compositions having hypolipidemic and/or hypoglycemic activity which contain a substituted naphthyloxyalkylaminobenzoic acid or salt or ester thereof.
表征谱图
-
氢谱1HNMR
-
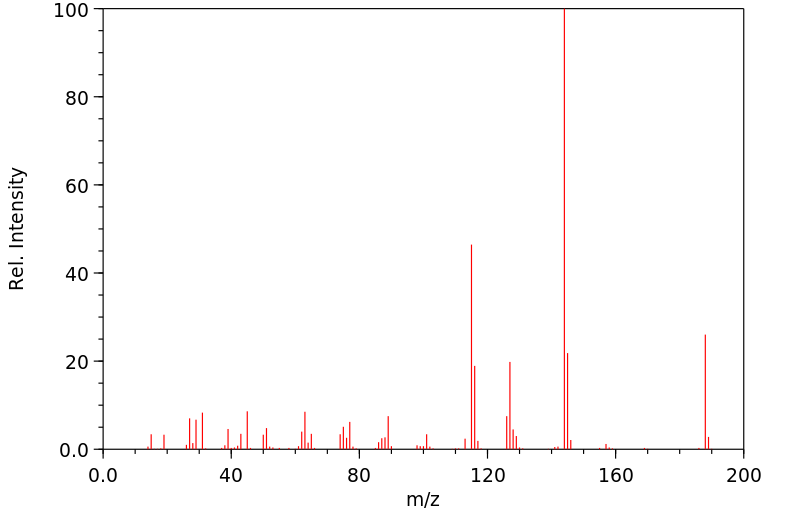
质谱MS
-
碳谱13CNMR
-
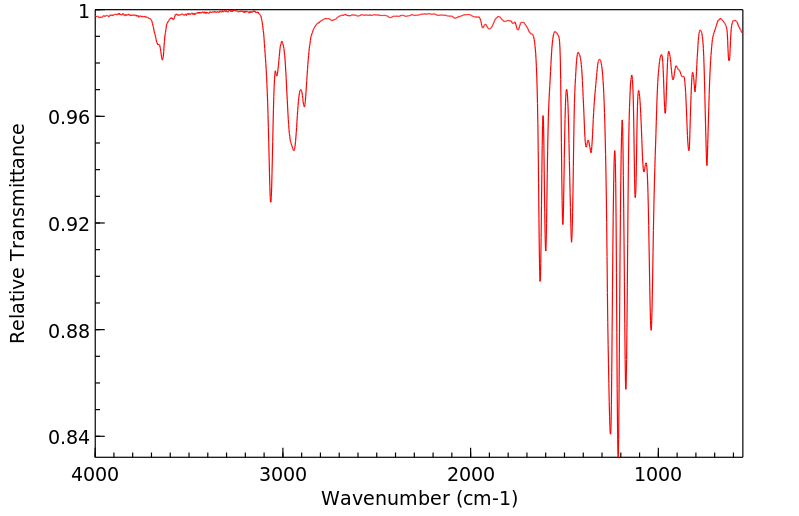
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮