2-(3-甲氧基苯氧基)丙酸 | 7309-52-6
中文名称
2-(3-甲氧基苯氧基)丙酸
中文别名
2-(3-甲氧基-苯氧基)-丙酸
英文名称
2-(3-Methoxy-phenoxy)-propionsaeure
英文别名
2-(3-Methoxyphenoxy)propanoic acid
CAS
7309-52-6
化学式
C10H12O4
mdl
MFCD01931912
分子量
196.203
InChiKey
QAVOEPJGKZAZIG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:93-94 °C
-
沸点:329.7±17.0 °C(Predicted)
-
密度:1.185±0.06 g/cm3(Predicted)
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:14
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:55.8
-
氢给体数:1
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
海关编码:2918990090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温且干燥环境下使用。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-(3-甲氧基苯氧基)丙酸乙酯 ethyl 2-(3-methoxyphenoxy)propanoate 141289-99-8 C12H16O4 224.257
反应信息
-
作为反应物:描述:2-(3-甲氧基苯氧基)丙酸 以15%的产率得到参考文献:名称:CAGNIANT P.; KIRSCH G., C. R. ACAD. SCI
, 1976, C 282, NO 21, 993-996 摘要:DOI: -
作为产物:描述:参考文献:名称:Birch et al., Journal of the Chemical Society, 1936, p. 1834,1837摘要:DOI:
文献信息
-
Access to Optically Enriched α-Aryloxycarboxylic Esters via Carbene-Catalyzed Dynamic Kinetic Resolution and Transesterification作者:Bin Liu、Runjiang Song、Jun Xu、Pankaj Kumar Majhi、Xing Yang、Song Yang、Zhichao Jin、Yonggui Robin ChiDOI:10.1021/acs.orglett.0c00748日期:2020.5.1Disclosed here is a carbene-catalyzed dynamic kinetic resolution and transesterification reaction for access to this class of molecules with up to 99% yields and 99:1 er values. Addition of a chiral carbene catalyst to the ester substrate leads to two diastereomeric azolium ester intermediates that can quickly epimerize to each other and thus allows for effective dynamic kinetic resolution to be realized
-
Chlorination of 2-Phenoxypropanoic Acid with NCP in Aqueous Acetic Acid: Using a Novel Ortho−Para Relationship and the Para/Meta Ratio of Substituent Effects for Mechanism Elucidation作者:Manuel A. P. Segurado、João Carlos R. Reis、Jaime D. Gomes de Oliveira、Senthamaraikannan Kabilan、Manohar ShanthiDOI:10.1021/jo0706224日期:2007.7.1beyond the aromatic ring. Activation enthalpies and entropies were estimated for substrates bearing the isoselective substituent in either ortho and para positions, being demonstrated that they are much different from the values for the parent substrate. The electrophilic attack on the phenolic oxygen atom by the protonated chlorinating agent is proposed as the rate-determining step, this step being followed对的(氧化chlorodehydrogenation测定速率常数[R ,小号)-2- phenoxypropanoic酸和九个邻- ,对- 10和五个间位取代的衍生物,使用(- [R ,小号)-1-氯-3-甲基-2,6-二苯基哌啶-4-酮(NCP)作为氯化剂。动力学是在伪一级反应条件下,相对于NCP,在5K(v / v)50%(v / v)的乙酸水溶液中,在高氯酸酸化的条件下,在298至318 K之间的5 K的温度间隔下进行的,衍生品。根据等动力学关系(IKR)分析了速率常数对温度的依赖性。对于在五个不同温度下研究的20个反应,等动力学温度估计为382 K,这表明水分子优先参与速率确定步骤。使用Hammett方程的四线性扩展分析了速率常数对间位和对位取代的依赖性。极性取代基效应的对位/间位比的参数λ估计为0.926,其静电模型表明在靠近苯氧基的氧原子附近带有电荷的活化络合物的形成。引入了一
-
Artemisinin (Qinghaosu) Derivatives, Their Preparation Methods And Their Use, And Pharmaceutical Compositions Containing The Same申请人:Li Ying公开号:US20080139642A1公开(公告)日:2008-06-12The invention provides a type of artemisinin derivatives having following structure I, its preparation method and use, as well as a pharmaceutical composition containing such artemisinin derivatives and its use. The artemisinin derivatives of the present invention and their pharmaceutical composition containing the artemisinin derivatives. have immunosuppressive activities and can be used more safely. The composition which comprises the artemisinin derivatives can be formulated into long-term dosage forms such as tablet, pellet and the like, and have wider productive and use value.
-
US7910750B2申请人:——公开号:US7910750B2公开(公告)日:2011-03-22
表征谱图
-
氢谱1HNMR
-
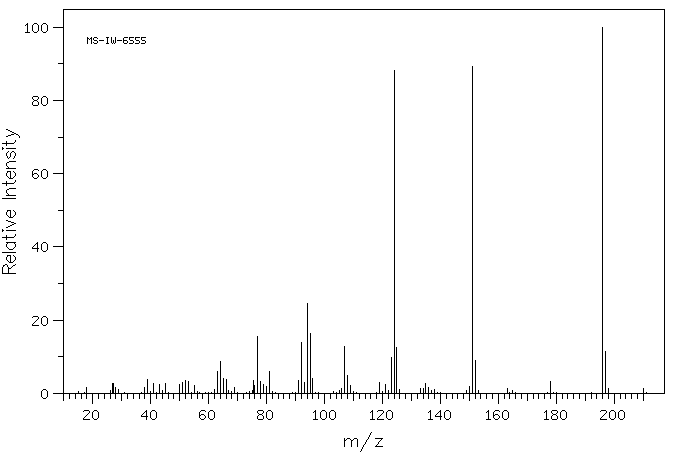
质谱MS
-
碳谱13CNMR
-
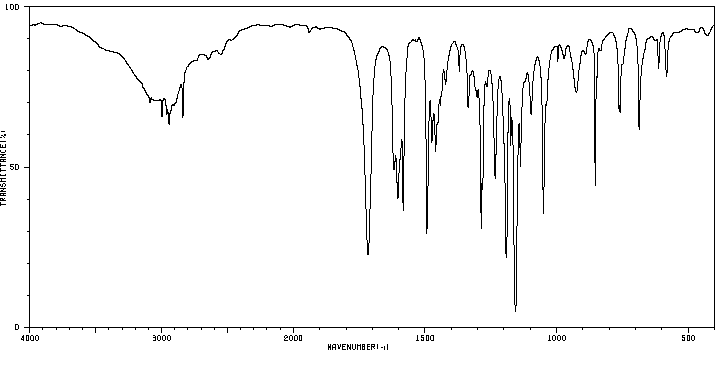
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫