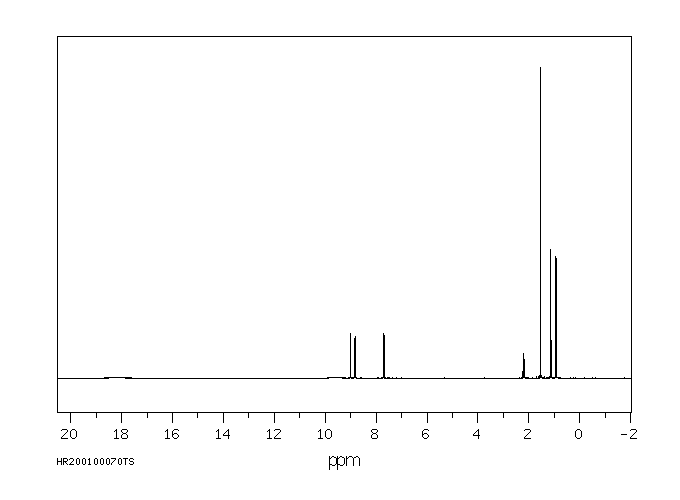
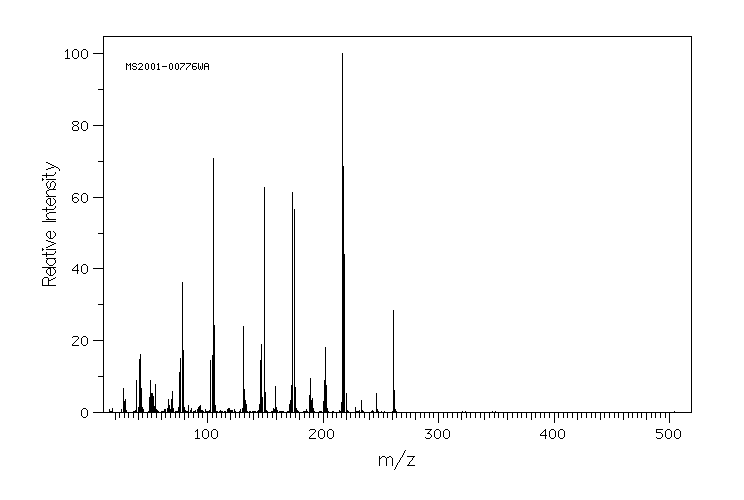
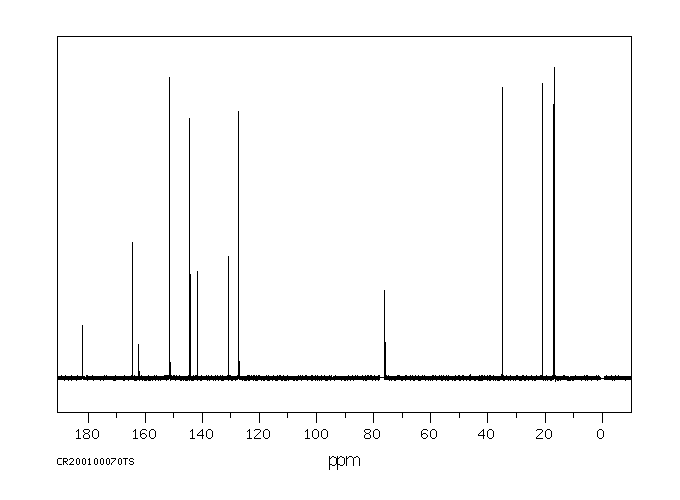
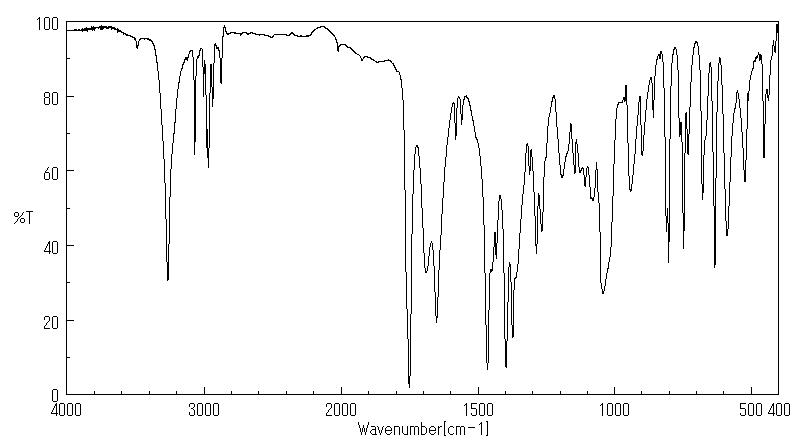
代谢
咪唑烟酸(未标记,活性成分99.5%,吡啶环上第6个碳上标记有(14)C,活性成分93.4%)。以大约9.5 mg/kg或924 mg/kg的单次灌胃剂量给予Sprague Dawley大鼠(每种性别/剂量5只),或者作为未标记的14天日剂量,随后给予单个标记剂量9.26 mg/kg。在一项初步研究中,对两只雄性和两只雌性大鼠单次灌胃给予10 mg/kg标记的咪唑烟酸,通过呼出气体研究了其排泄情况。玉米油用作所有口服治疗的载体。另一项研究中,五只雄性和五只雌性大鼠通过静脉注射接受了9.94 mg/kg的咪唑烟酸。代谢物特征研究显示,几乎所有测试物质都以原形排出。在处理过的大鼠的尿液或粪便中检测到两种次要代谢物CL 252,974(2-((1-羧基-1,2-二甲基丙基)-羧基)-烟酸)和CL 60,032(2-羧基-烟酸),但它们的总贡献小于给药剂量的0.5%。还分离出多达12种额外的未识别代谢物,但它们构成给药剂量的< 3%。根据结果,咪唑烟酸的有限代谢通过水解形成2-羰基衍生物:CL 252,974和CL 60,032。
Imazapyr, (unlabeled, 99.5% ai, (14)C-labeled at the 6-carbon on the pyridine ring, 93.4% ai). was administered to Sprague Dawley rats (5/sex/dose) as a single gavage dose of approximately 9.5 mg/kg or 924 mg/kg or as 14-daily doses of unlabeled followed by a single labeled dose of 9.26 mg/kg. Excretion via expired air was examined in a pilot study where two male and two female rats were given a single gavage dose of 10 mg/kg labeled imazapyr. Corn oil was used as the vehicle for all oral treatments. An additional study with five male and five female rats that received 9.94 mg/kg imazapyr by intravenous injection was also done. Metabolite characterization studies show that essentially all of the test material was excreted unchanged. Two minor metabolites CL 252,974 (2-((1-carbamoyl-1,2-dimethylpropyl)- carbamoyl)-nicotinic acid) and CL 60,032 (2-carbamoyl-nicotinic acid) were detected in the urine or feces of treated rats; however, their contribution combined was < 0.5% of the administered dose. Up to 12 additional unidentified metabolites were isolated, but they constituted < 3% of the administered dose. Based on the results, ... what limited metabolism of imazapyr occurs, proceeds through hydrolysis to form the 2-carbonyl derivatives: CL 252,974 and CL 60,032.
来源:Hazardous Substances Data Bank (HSDB)