2-(环己氨基甲基)吡啶 | 68339-45-7
中文名称
2-(环己氨基甲基)吡啶
中文别名
2-(环己基氨基甲基)吡啶
英文名称
N-cyclohexyl-N-(pyridin-2-ylmethyl)amine
英文别名
2-(Cyclohexylaminomethyl)pyridine;N-(pyridin-2-ylmethyl)cyclohexanamine
CAS
68339-45-7
化学式
C12H18N2
mdl
MFCD01135534
分子量
190.288
InChiKey
KUBDCHSZNMFKFV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
密度:1.01
-
稳定性/保质期:
在常温常压下稳定,应远离氧化剂。
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:14
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.583
-
拓扑面积:24.9
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2933399090
-
储存条件:存放在密封容器内,并置于阴凉、干燥处。务必远离氧化剂和热源,避光保存。
SDS
2-(环己氨基甲基)吡啶
模块 1. 化学品
产品名称: 2-(Cyclohexylaminomethyl)pyridine
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-(环己氨基甲基)吡啶
百分比: >98.0%(GC)(T)
CAS编码: 68339-45-7
俗名: N-Cyclohexyl-2-Picolylamine , N-(2-Pyridylmethyl)cyclohexylamine
2-(环己氨基甲基)吡啶
模块 3. 成分/组成信息
分子式: C12H18N2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
气敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
2-(环己氨基甲基)吡啶
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 微浅黄色-黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.01
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
2-(环己氨基甲基)吡啶
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 2-(Cyclohexylaminomethyl)pyridine
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-(环己氨基甲基)吡啶
百分比: >98.0%(GC)(T)
CAS编码: 68339-45-7
俗名: N-Cyclohexyl-2-Picolylamine , N-(2-Pyridylmethyl)cyclohexylamine
2-(环己氨基甲基)吡啶
模块 3. 成分/组成信息
分子式: C12H18N2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
气敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
2-(环己氨基甲基)吡啶
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 微浅黄色-黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.01
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
2-(环己氨基甲基)吡啶
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N-[[3-[[cyclohexyl(pyridin-2-ylmethyl)amino]methyl]-1H-pyrazol-5-yl]methyl]-N-(pyridin-2-ylmethyl)cyclohexanamine 1295578-63-0 C29H40N6 472.677
反应信息
-
作为反应物:描述:2-(环己氨基甲基)吡啶 在 platinum(IV) oxide 氢气 作用下, 以 溶剂黄146 为溶剂, 以70%的产率得到2-(N-cyclohexylaminomethyl)piperidine参考文献:名称:具有桥头氮 52 的化合物——2-烷基全氢咪唑并[3,4-a]吡啶的核磁共振谱和立体化学摘要:与全氢恶唑并[3,4-a]吡啶和全氢噻唑并[3,4-a]吡啶相反,它们在室温下在CDCl3溶液中采用平衡,其中含有约70%的与O-或S-平衡的互变构象异构体在顺式融合的构象异构体中,发现 2-烷基全氢咪唑并[3,4-a]吡啶采用含有>98% 的反式融合构象异构体的平衡。2-甲基全氢咪唑并[3,4-a]吡啶的NMR参数与1,2-二甲基全氢咪唑并[3,4-a]吡啶的两种异构体的NMR参数的比较表明前一种化合物在两种反式融合构象异构体之间达到平衡,大约 83% 的构象包含氮孤对电子的反式排列。这些观察结果是根据广义异头效应来解释的。DOI:10.1002/mrc.1260250808
-
作为产物:描述:N-(2-pyridinylmethylene)cyclohexylamine 在 sodium tetrahydroborate 作用下, 以 甲醇 为溶剂, 反应 3.0h, 生成 2-(环己氨基甲基)吡啶参考文献:名称:具有桥头氮 52 的化合物——2-烷基全氢咪唑并[3,4-a]吡啶的核磁共振谱和立体化学摘要:与全氢恶唑并[3,4-a]吡啶和全氢噻唑并[3,4-a]吡啶相反,它们在室温下在CDCl3溶液中采用平衡,其中含有约70%的与O-或S-平衡的互变构象异构体在顺式融合的构象异构体中,发现 2-烷基全氢咪唑并[3,4-a]吡啶采用含有>98% 的反式融合构象异构体的平衡。2-甲基全氢咪唑并[3,4-a]吡啶的NMR参数与1,2-二甲基全氢咪唑并[3,4-a]吡啶的两种异构体的NMR参数的比较表明前一种化合物在两种反式融合构象异构体之间达到平衡,大约 83% 的构象包含氮孤对电子的反式排列。这些观察结果是根据广义异头效应来解释的。DOI:10.1002/mrc.1260250808
文献信息
-
A Phosphine-Free Manganese Catalyst Enables Stereoselective Synthesis of (1 + <i>n</i>)-Membered Cycloalkanes from Methyl Ketones and 1,<i>n</i>-Diols作者:Akash Jana、Kuhali Das、Abhishek Kundu、Pradip Ramdas Thorve、Debashis Adhikari、Biplab MajiDOI:10.1021/acscatal.9b05567日期:2020.2.21Herein, we report the stereoselective synthesis of (1 + n)-membered cycloalkane from methyl ketone and 1,n-diol. A manganese(I) complex bearing a phosphine-free ligand catalyzed the reaction, which involved the formation of two C–C bonds via a sequence of intermolecular- and intramolecular-borrowing hydrogenation reactions. It produces 2 mol of water as the sole byproduct, making the process atom economical
-
Chromotropism in halo-bridged dimers. Structural characterization of bis(μ-halo)bis(halo N-(pyridin-2-ylmethyl)cyclohexanamine copper(II))作者:Hamid Golchoubian、Samira NateghiDOI:10.1080/00958972.2016.1227972日期:2016.11.1position of the copper(II) being occupied by the bridging chloride anion which is equatorial to the other copper ion, forming a dimeric copper(II) complex. The chromotropic properties of both complexes, including solvato-, thermo-, and halochromism, were investigated. The complexes show reversible thermochromism in solution which is irreversible in the solid state. It was found that the solvatochromism is摘要 两种双核铜 (II) 配合物 [LCu(μ-Cl)Cl]2 (DMF) (1) 和 [LCu(μ-Br)Br]2 (2),具有双齿配体 N-(pyridin-2)合成了-基甲基)环己胺,L,并通过物理化学和光谱(IR,UV-vis)数据进行表征。1 的晶体结构分析表明,两个铜 (II) 离子都处于扭曲的四角锥 N2Cl3 环境中,铜 (II) 的顶端位置被与另一个铜离子赤道的桥接氯阴离子占据,形成一个二聚铜(II)配合物。研究了这两种配合物的变色特性,包括溶剂化、热和卤化变色。该配合物在溶液中表现出可逆的热致变色现象,而在固态时则不可逆。发现溶剂化显色是由于结构变化以及配合物空位的溶剂化所致。通过可见吸收光谱在 1-11 的 pH 范围内研究了它们的卤致变色特性。颜色从蓝色变为绿色并变为无色是由于配体的去质子化和质子化。
-
Anchored Palladium Complex‐Generated Clusters on Zirconia: Efficiency in Reductive <i>N</i> ‐Alkylation of Amines with Carbonyl Compounds under Hydrogen Atmosphere作者:Zhenzhong Zhang、Takuya Ikeda、Haruno Murayama、Tetsuo Honma、Makoto Tokunaga、Yukihiro MotoyamaDOI:10.1002/asia.202101243日期:2022.4generation method of palladium clusters and catalytic reductive N-alkylation of amines with carbonyl compounds are reported. The active Pd species are formed by treatment of highly dispersed molecular palladium complexes with atmospheric hydrogen, and the present catalyst system shows a broad substrate scope and functional group tolerance with respect to both amines and carbonyl compounds.
-
Rhodium catalysts with cofactor mimics for the biomimetic reduction of CN bonds作者:Jie Tang、Wenjin Dong、Fushan Chen、Li Deng、Mo XianDOI:10.1039/d1cy00904d日期:——from a rhodium center to imine substrates in a biomimetic way. Under both transfer hydrogenation and reductive amination reaction conditions, the catalyst exhibited good selectivity towards CN bonds. With the catalyst, 34 imines were transfer hydrogenated to corresponding amines and a key intermediate of retigabine was prepared via reductive amination in a greener way. According to the NMR observations
-
Synthesis and characterization of aminopyridine iron(<scp>ii</scp>) chloride catalysts for isoprene polymerization: sterically controlled monomer enchainment作者:Chuyang Jing、Liang Wang、Qaiser Mahmood、Mengmeng Zhao、Guangqian Zhu、Xianhui Zhang、Xiaowu Wang、Qinggang WangDOI:10.1039/c9dt00452a日期:——analysis: Fe3Me and Fe11Me adopted distorted tetrahedral geometries in the solid state while Fe4H and Fe7H were found in dimeric or polymeric forms respectively in which chlorides acted as bridging ligands. The catalytic capacities of these iron complexes were investigated for isoprene polymerization. Upon activation with a MAO cocatalyst, the catalytic activities of complexes varied as a function of the steric在这项研究中,一系列的2-R-6-(1-(烷基氨基)甲基)吡啶-铁络合物[烷基:(CPh 3)Fe1 H ; (CHPh 2)Fe2 H ; (CHPh 2)Fe3 Me ; (CHMePh)FE4 ħ ; (CH 2 Ph)Fe5 H ; (CHMe 2)Fe6 H ; (C 6 H 11)Fe7 H ; (CH 2(4-OMe)Ph)Fe8 H ; (CH 2(4-CF 3)Ph)Fe9 H ; (CH2(2,4,6-Me 3)Ph) Fe10 H ; 合成了(CH 2 Ph) Fe11 Me ],并通过ATR-IR光谱,HRMS光谱和元素分析对其进行了很好的表征。此外,的Fe3我, FE4 ħ, Fe7 ħ和FE11我用X射线衍射分析:的Fe3我和FE11我通过了在固态扭曲四面体几何形状而FE4 ħ和Fe7 ħ分别以二聚或聚合形式发现氯,其中氯化物充当桥连配体。研究了这些铁络合物对异
表征谱图
-
氢谱1HNMR
-
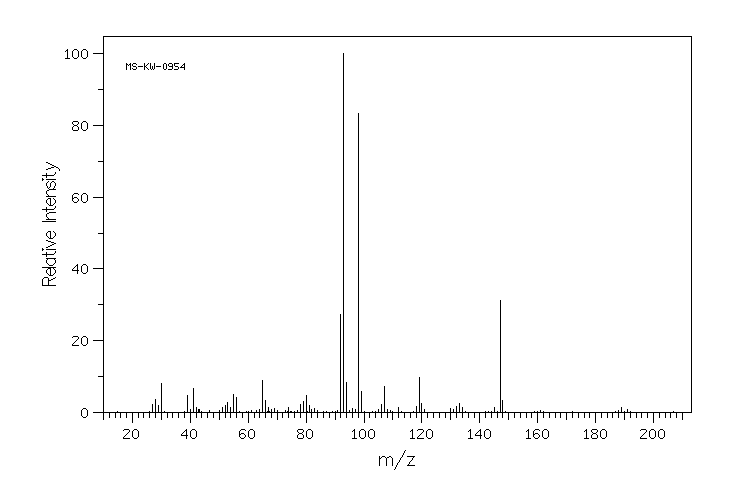
质谱MS
-
碳谱13CNMR
-
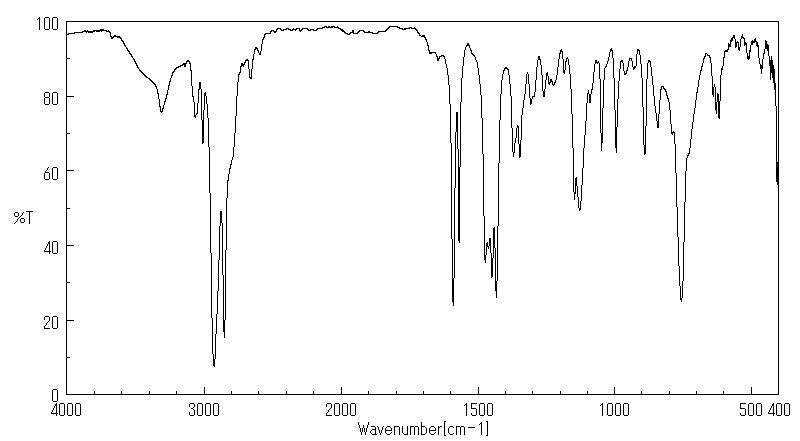
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-