玉红省 | 197-61-5
中文名称
玉红省
中文别名
——
英文名称
rubicene
英文别名
Rubicen
CAS
197-61-5
化学式
C26H14
mdl
——
分子量
326.397
InChiKey
FMKFBRKHHLWKDB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:305 °C
-
沸点:399.43°C (rough estimate)
-
密度:1.2006 (estimate)
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):7.7
-
重原子数:26
-
可旋转键数:0
-
环数:7.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
安全说明:S24/25
-
海关编码:2901299090
-
储存条件:密封储存,应存放在阴凉、干燥的库房中。
SDS
| Name: | Rubicene Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 197-61-5 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 197-61-5 | Rubicene | 205-899-5 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam. Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 197-61-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: red
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C26H14
Molecular Weight: 326.098
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 197-61-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Rubicene - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 197-61-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 197-61-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 197-61-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:参考文献:名称:Sachweh, Volker; Langhals, Heinz, Chemische Berichte, 1990, vol. 123, # 10, p. 1981 - 1987摘要:DOI:
-
作为产物:参考文献:名称:双离子电池,使用二氨基二茂铁作为负离子正电极材料摘要:为了促进分子离子可充电电池的研究,已设计并制备了一种新型的非聚合阴离子插入电极材料:5,12-二氨基红花酮(DARb)。红宝石分子的非极性核心结构已与两个氨基偶联,以产生对极性溶剂(如常见的基于碳酸盐的电池电解质)具有低亲和力的原始共轭伯二胺。已使用六种不同的电解质配方(在溶剂(PC,EC-DMC)和锂盐(LiPF 6,LiClO 4,LiTFSI)中)使用六种不同的电解质配方在双离子电池配置(相对于Li)中探测了这种有机分子的电化学反应性。当使用1 M LiPF时,这种二氨基红花环材料系统地显示出可逆的电活性和有希望的性能6在EC:DMC(1:1体积%)作为电解液,如〜3.4 V,相对于的平均电位锂+ /锂0,115毫安·g的初始容量- 1和良好的容量保持比60个循环,无任何优化。DOI:10.1016/j.elecom.2016.09.002
文献信息
-
Studies on organophosphorus compounds—XL作者:S. Scheibye、R. Shabana、S.-O. Lawesson、C. RømmingDOI:10.1016/0040-4020(82)85078-3日期:1982.1Cyclohexanone and cyclopentanone react with 2,4 - bis - 4 - methoxyphenyl) - 1,3,2,4 - dithiadiphosphetane 2,4-disulfide (lawesson Reagent (LR) at 80° with formation of new spiro - 1,3,5,2 - trithiaphosphorines 1 and 2, respectively. 2-Methyl and 2-phenylcyclohexanone also react with LR at 80° producing the enethiols 3 and 4, which on storage are transformed into the sulfides 5 and 6. Unsaturated cyclohexanones
-
A New Suzuki−Heck-Type Coupling Cascade: Indeno[1,2,3]-Annelation of Polycyclic Aromatic Hydrocarbons作者:Hermann A. Wegner、Lawrence T. Scott、Armin de MeijereDOI:10.1021/jo020367h日期:2003.2.1Under palladium catalysis, o-bromobenzeneboronic acid can be coupled with 1-bromonaphthalene (6) and with oligocyclic bromoarenes to furnish indeno-annelated polycyclic aromatic hydrocarbons 1-4 and 25 in a single operation in moderate to good yields (27-87%). Alternatively, o-dibromoarenes and 1,2-dibromocycloalkenes can be cross-coupled with 1-naphthaleneboronic acid under the same conditions to
-
Probing Mechanisms of Aryl–Aryl Bond Cleavages under Flash Vacuum Pyrolysis Conditions作者:Edward A. Jackson、Xiang Xue、Hee Yeon Cho、Lawrence T. ScottDOI:10.1071/ch14171日期:——radicals; (2) hydrogen atom attachment to an ipso-carbon atom of the biaryl followed by C–C bond cleavage; (3) direct homolysis; and (4) loss of a fragment as an aryne. None of these mechanisms by itself successfully accommodates all of the experimental facts. The data suggest that aryl–aryl bond cleavages under FVP conditions involve at least two different mechanistic pathways and that the relative几种联芳基已在1100°C和0.8-0.9 hPa的条件下进行了快速真空热解(FVP)。报告了9-苯基蒽(1),2-溴代联苯(5),联苯(8),1,10-二苯基蒽(12),9-(2-萘基)蒽(17)和9的FVP产品组成,9'-双蒽基(20)。实验结果已用于评估在这些条件下芳基-芳基键断裂的四种可能的机理途径:(1)取代苯基的“爆炸”;(2)氢原子与ipso的连接-联芳基的碳原子,然后进行CC键断裂;(3)直接匀浆;(4)丢失作为芳烃的片段。这些机制本身都不能成功地适应所有实验事实。数据表明,FVP条件下的芳基-芳基键裂解涉及至少两个不同的机理途径,竞争途径的相对贡献可能从一个联芳基变化到另一个。
-
Microwave Flash Pyrolysis: C9H8 Interconversions and Dimerisations作者:Aida Ajaz、Alicia C. Voukides、Katharine J. Cahill、Rajesh Thamatam、Sarah L. Skraba-Joiner、Richard P. JohnsonDOI:10.1071/ch14238日期:——of bi-indene and by computational models. Benzo[a]anthracene and benzo[c]phenanthrene are minor products in these reactions. These are shown to arise from pyrolysis of chrysene under the same MFP conditions. MFP reaction of fluorene gave primarily bi-fluorene, bifluorenylidene, and dibenzochrysene, the latter derived from a known Stone–Wales rearrangement.已经通过密封管微波闪速热解(MFP)研究了2-乙炔基甲苯,茚,芴和相关化合物的热解,并与通过密度泛函理论(DFT)计算对推定的机理途径进行了建模。在MFP技术中,样品与石墨混合,并在氮气气氛下的石英反应管中经受强微波功率(150-300 W)。2-乙炔基甲苯的MFP反应主要产生茚,其为Roger Brown重排(1,2-H转变为亚乙烯基)的产物,然后插入。另一产物是,这可能是由于茚中氢原子损失,然后进行二聚作用所致。双茚的热解和计算模型支持了二聚双茚结构的中间性。苯并[ a在这些反应中,蒽和苯并[ c ]菲是次要产物。这些是由于在相同的MFP条件下进行的热裂解而产生的。芴的MFP反应主要产生联芴,联芴基和二苯并芴,后者衍生自已知的Stone-Wales重排。
-
Proton-Catalyzed, Silane-Fueled Friedel-Crafts Coupling of Fluoroarenes作者:Oliver Allemann、Simon Duttwyler、Paola Romanato、Kim K. Baldridge、Jay S. SiegelDOI:10.1126/science.1202432日期:2011.4.29carbon-carbon bonds. The venerable Friedel-Crafts reaction appends alkyl or acyl groups to aromatic rings through alkyl or acyl cation equivalents typically generated by Lewis acids. We show that phenyl cation equivalents, generated from otherwise unreactive aryl fluorides, allow extension of the Friedel-Crafts reaction to intramolecular aryl couplings. The enabling feature of this reaction is the exchange在Friedel-Crafts 中加入F Friedel-Crafts 类反应是有机化学中最古老和应用最广泛的反应之一,它在芳环和各种非芳族取代基(如烷基)之间形成碳-碳键。通常,金属络合物用于活化这些取代基的氯化或溴化前体,但通过使用基于硅的试剂来活化氟化前体,Allemann 等人。(p. 574) 将反应扩展到两个不同芳香位点的偶联,从而有效地形成复杂的多环结构。该方法依赖于硅-氟键的异常强度作为驱动力。硅-氟键的形成扩大了可用于形成碳-碳键的反应的化合物范围。古老的 Friedel-Crafts 反应通过通常由路易斯酸生成的烷基或酰基阳离子等价物将烷基或酰基附加到芳环上。我们表明,由其他不反应的芳基氟化物产生的苯基阳离子等价物允许将 Friedel-Crafts 反应扩展到分子内芳基偶联。该反应的启动特征是碳-氟交换为硅-氟键焓;该反应由中间体甲硅烷基阳离子激活。催化量的质子或甲硅
表征谱图
-
氢谱1HNMR
-
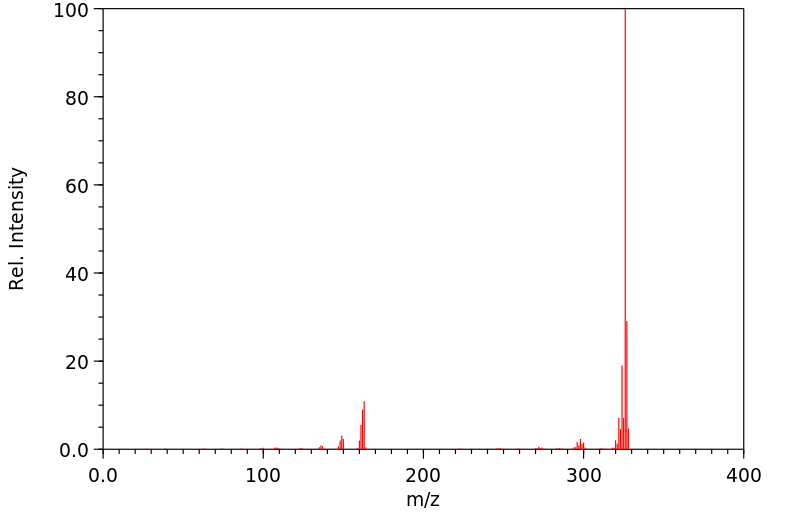
质谱MS
-
碳谱13CNMR
-
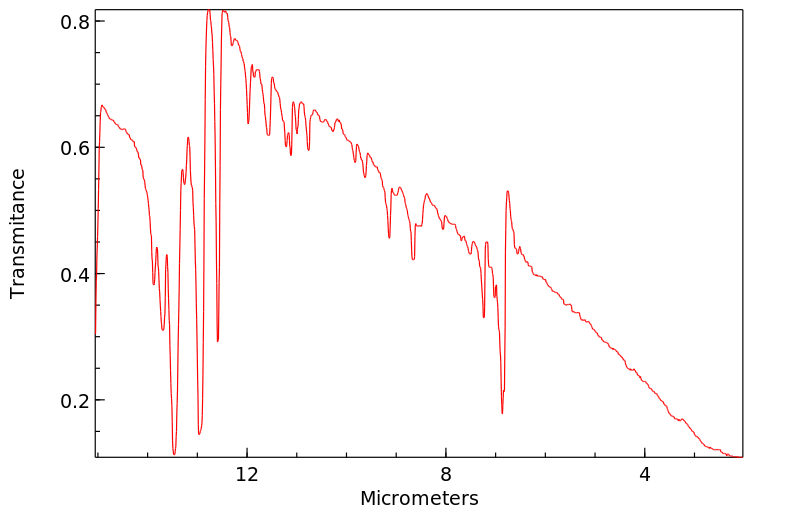
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62