3-phenyl-3a,6a-dihydrofuro<2,3-d>isoxazole
中文名称
——
中文别名
——
英文名称
3-phenyl-3a,6a-dihydrofuro<2,3-d>isoxazole
英文别名
cis-3a,6a-dihydro-3-phenylfuro[2,3-d]isoxazole;(3As,6as)-3-phenyl-3a,6a-dihydro-furo[2,3-d]isoxazole;(3aS,6aS)-3-phenyl-3a,6a-dihydrofuro[2,3-d][1,2]oxazole
CAS
——
化学式
C11H9NO2
mdl
——
分子量
187.198
InChiKey
BGBBXGVLPOLRIX-GXSJLCMTSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:14
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.18
-
拓扑面积:30.8
-
氢给体数:0
-
氢受体数:3
反应信息
-
作为反应物:描述:参考文献:名称:通过异恶唑啉合成氨基糖:一氧化氮-呋喃加合物是异恶唑啉路线中通往新型氨基糖衍生物的关键中间体摘要:提出了新的1,3-偶极一氧化氮环化成难于吸附的偶极亲和性呋喃和2-甲基呋喃的新形式。呋喃并恶唑啉加合物6和7已显示出代表新型C 6和C 7氨基糖衍生物衍生物的高度先进而通用的前体,可通过随后的高度立体选择性修饰(添加HO / OCH 3或HO / OH)和/或LiAlH 4还原。DOI:10.1016/s0040-4020(01)96705-5
-
作为产物:描述:呋喃 、 benzohydroximoyl chloride 在 三氟化硼乙醚 、 三乙胺 作用下, 反应 240.0h, 以48%的产率得到3-phenyl-3a,6a-dihydrofuro<2,3-d>isoxazole参考文献:名称:通过异恶唑啉合成氨基糖:一氧化氮-呋喃加合物是异恶唑啉路线中通往新型氨基糖衍生物的关键中间体摘要:提出了新的1,3-偶极一氧化氮环化成难于吸附的偶极亲和性呋喃和2-甲基呋喃的新形式。呋喃并恶唑啉加合物6和7已显示出代表新型C 6和C 7氨基糖衍生物衍生物的高度先进而通用的前体,可通过随后的高度立体选择性修饰(添加HO / OCH 3或HO / OH)和/或LiAlH 4还原。DOI:10.1016/s0040-4020(01)96705-5
文献信息
-
Preparation, Properties, and Chemical Reactivity of α-Nitrosulfoximines, Chiral Analogues of α-Nitrosulfones作者:Peter A. Wade、Hung Le、Nayan V. AminDOI:10.1021/jo010924k日期:2002.5.1Racemic N-methyl-S-(nitromethyl)-S-phenylsulfoximine (2) was prepared in 87% yield via alkaline nitration of N,S-dimethyl-S-phenylsulfoximine. Optically active N-methyl-S-(nitromethyl)-S-phenylsulfoximine (both enantiomers) was prepared in similar fashion. Reaction of racemic 2 with p-chlorophenyl isocyanate and a catalytic quantity of triethylamine in the presence of furan afforded dihydrofuroisoxazole
-
Synthesis of amino sugars via isoxazolines the concept and one application: nitrile oxide/furan adducts作者:Ingrid Müller、Volker JägerDOI:10.1016/s0040-4039(00)85711-1日期:1982.1
表征谱图
-
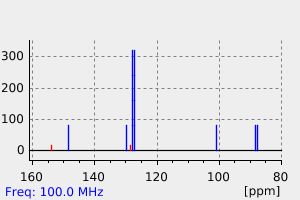
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫