2-庚基-1,3-二氧戊环 | 4359-57-3
中文名称
2-庚基-1,3-二氧戊环
中文别名
辛醛乙二醇缩醛;辛醛丙二醇缩醛
英文名称
2-heptyl-1,3-dioxolane
英文别名
——
CAS
4359-57-3
化学式
C10H20O2
mdl
MFCD00216992
分子量
172.268
InChiKey
LWJBQKKNODYJEI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:115-116 °C(Press: 45 Torr)
-
密度:0.902±0.06 g/cm3(Predicted)
-
LogP:3.140 (est)
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:12
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2932999099
-
储存条件:存放于惰性气体中,并避免接触湿气(以免发生分解)。
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Facile Conversion of Acetals to Nitriles.摘要:脂肪族和芳香族缩醛在回流的无水乙醇中,可通过与盐酸羟胺反应,容易且高效地转化为相应的腈类化合物。DOI:10.1248/cpb.41.2042
-
作为产物:描述:参考文献:名称:Combination of NH2OH·HCl and NaIO4: an effective reagent for molecular iodine-free regioselective 1,2-difunctionalization of olefins and easy access of terminal acetals摘要:我们展示了我们的氧化试剂的新应用,这是NH2OH·HCl和NaIO4的组合,在烯烃的第一种广义区域选择性1,2-二官能团化中的应用。DOI:10.1039/c5ra11092k
文献信息
-
A Novel, Efficient, and Selective Cleavage of Acetals Using Bismuth(III) Chloride作者:Gowravaram Sabitha、R. Satheesh Babu、E. Veakata Reddy、J. S. YadavDOI:10.1246/cl.2000.1074日期:2000.9Treatment of acetals with bismuth(III) chloride in methanol provides a simple, convenient and chemoselective process for deprotection, and the parent aldehyde or ketone was obtained in high yield. The acetals have been cleaved selectively in the presence of silyl, benzyl and tetrahydropyranyl ethers.
-
Robust acidic pseudo-ionic liquid catalyst with self-separation ability for esterification and acetalization作者:Yingxia Shi、Xuezheng LiangDOI:10.1007/s11696-019-00693-1日期:2019.6The novel acidic pseudo-ionic liquid catalyst with self-separation ability has been synthesized through the quaternization of triphenylphosphine and the acidification with silicotungstic acid. The pseudo-IL showed high activities for the esterification with average conversions over 90%. The pseudo-IL showed even higher activities for acetalization than traditional sulfuric acid. The homogeneous catalytic
-
A Simple, Efficient and General Procedure for Acetalization of Carbonyl Compounds and Deprotection of Acetals under the Catalysis of Indium(III) Chloride作者:Brindaban C. Ranu、Ranjan Jana、Sampak SamantaDOI:10.1002/adsc.200303154日期:2004.3Indium(III) chloride efficiently catalyzes the protection of a variety of aldehydes and ketones to their corresponding 1,3-dioxolanes and dialkyl acetals in refluxing cyclohexane. On the other hand, deprotection of acetals is also achieved in refluxing aqueous methanol under the catalysis of indium(III) chloride.
-
Anhydrous Iron(III) Chloride Dispersed on Silica Gel; III.<sup>1,2</sup>A Convenient and Mild Reagent for Deacetalization in Dry Medium
-
A New Ready, High-Yielding, General Procedure for Acetalization of Carbonyl Compounds作者:Romualdo Caputo、Carla Ferreri、Giovanni PalumboDOI:10.1055/s-1987-27955日期:——Carbonyl compounds are smoothly and rapidly acetalized by treatment with alcohols, in anhydrous acetonitrile, in the presence of polystyryl diphenyl phosphine - iodine complex as catalyst. Open and cyclic acetals, including 1,3-dioxolanes, 1,3-oxathiolanes, and 1,3-dithiolanes, of miscellaneous aldehydes and ketones have been successfully prepared in this way. The isolation of the product is very easily performed, by simple filtration of the polymer-linked phosphine oxide which is formed in the reaction.
表征谱图
-
氢谱1HNMR
-
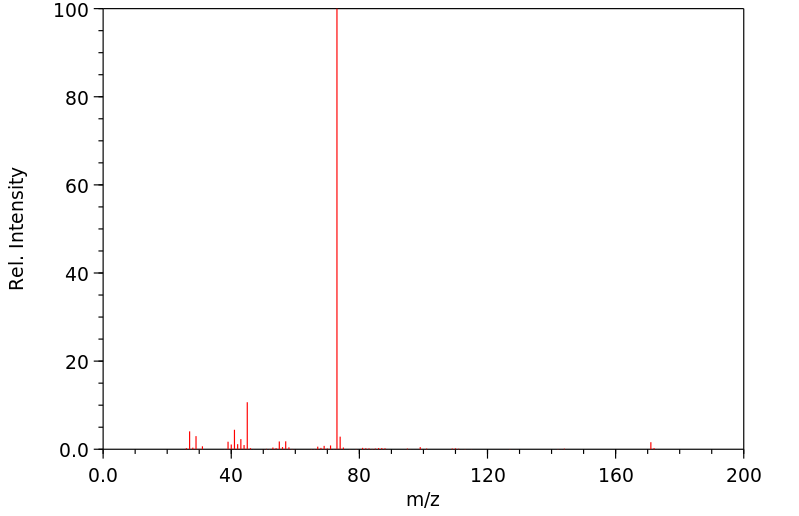
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-甲基-4-叔-丁基-1,3-二氧戊环
过氧竹红菌素
辛醛丙二醇缩醛
碘丙甘油
甜瓜醛丙二醇缩醛
甘油缩甲醛
甘油缩甲醛
环辛基甲醛乙烯缩醛
环戊二烯内过氧化物
环己丙胺,1-(1,3-二噁戊环-2-基)-
环丙羧酸,2-乙酰基-,甲基酯,(1R-顺)-(9CI)
氯乙醛缩乙二醇
柠檬醛乙二醇缩醛
异戊醛丙二醇缩醛
异丁醛-丙二醇缩醛
奥普碘铵
多米奥醇
多效缩醛
壬醛丙二醇缩醛
四吖戊啶,5-(1-吡咯烷基)-
亲和素
二氰苯乙烯酮乙烯缩醛
乙酮,1-(2-环辛烯-1-基)-,(-)-(9CI)
乙基1,3-二氧戊环-4-羧酸酯
丙炔醛乙二醇缩醛
三甲基-[(2-甲基-1,3-二氧戊环-4-基)甲基]铵碘化物
三氟乙烯臭氧化物
三丁基(1,3-二恶烷-2-基甲基)溴化鏻
[2-(2-碘乙基)-1,3-二氧戊环-4-基]甲醇
6,8-二氧杂二螺[2.1.4.2]十一烷
6,7-二氧杂双环[3.2.1]辛-2-烯-8-羧酸
5H,8H-呋喃并[3,4:1,5]环戊二烯并[1,2-d]-1,3-二噁唑(9CI)
5-过氧化氢基-5-甲基-1,2-二恶烷-3-酮
5-嘧啶羧酸,4-(2-呋喃基)-1,2,3,4-四氢-6-甲基-2-羰基-,1-甲基乙基酯
5-(哌嗪-1-基)苯并呋喃-2-甲酰胺
5-(1,3-二氧杂烷-2-基)呋喃-2-磺酰氯
5-(1,3-二氧戊环-2-基)戊腈
5,5-二羟基戊醛
4a-乙基-2,4a,5,6,7,7a-六氢-4-(3-羟基苯基)-1-甲基-1H-1-吡喃并英并啶
4-硝基-4-丙基辛醛乙烯缩醛
4-甲基-4-硝基辛醛乙烯缩醛
4-甲基-2-戊基-1,3-二氧戊环
4-甲基-2-十一烷基-1,3-二氧戊环
4-甲基-2-[(1E)-1-戊烯-1-基]-1,3-二氧戊环
4-甲基-2-(三氯甲基)-1,3-二氧戊环
4-甲基-2-(2-(甲硫基)乙基)-1,3-二氧戊环
4-甲基-2-(1-丙烯基)-1,3-二氧戊环
4-甲基-1,3-二氧戊环
4-烯丙基-4-甲基-2-乙烯基-1,3-二氧戊环
4-溴-3,5,5-三甲基二氧戊环-3-醇