2-异硫氰酸辛酯 | 21663-52-5
中文名称
2-异硫氰酸辛酯
中文别名
——
英文名称
2-isothiocyanatooctane
英文别名
2-octyl isothiocyanate;1-methyl-heptyl isothiocyanate;(α-Methyl-n-heptyl)-isothiocyanat;(α-Methyl-n-heptyl)-senfoel;1-Methyl-heptylisothiocyanat;sek. n-Octyl-senfoel
CAS
21663-52-5;69626-80-8
化学式
C9H17NS
mdl
——
分子量
171.307
InChiKey
RXAAZXUXIQEORL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.9
-
重原子数:11
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.89
-
拓扑面积:44.4
-
氢给体数:0
-
氢受体数:2
SDS
反应信息
-
作为反应物:参考文献:名称:Jahn, Monatshefte fur Chemie, 1882, vol. 3, p. 172摘要:DOI:
-
作为产物:描述:参考文献:名称:Isothiocyanates (ITCs) 1-(Isothiocyanatomethyl)-4-phenylbenzene and 1-Isothiocyanato-3,5-bis(trifluoromethyl)benzene—Aldehyde Dehydrogenase (ALDH) Inhibitors, Decreases Cisplatin Tolerance and Migratory Ability of NSCLC摘要:非小细胞肺癌(NSCLC)的主要治疗模式之一是以顺铂为基础的化疗。然而,获得顺铂耐药性仍然是一个主要问题。现有的化疗方案通常对表达醛脱氢酶(ALDH)的癌细胞无效。因此,迫切需要针对ALDH阳性癌细胞的治疗方法。本研究比较了36种结构多样的异硫氰酸酯(ITC)与ALDH抑制剂二硫化物(DSF)对NSCLC细胞的抗癌性能。使用AutoDockTools评估它们与ALDH同工酶和ABC蛋白的潜在亲和力,以选择出三种对所有测试蛋白的亲和力最强的化合物。所选的ITC对NSCLC细胞的存活率没有影响(在测试浓度下),但显著降低了顺铂耐药变异株A549CisR和晚期(第四期)NSCLC细胞系H1581的顺铂耐受性。此外,长期补充ITC 1-异硫氰酸甲基-4-苯基苯烷可逆转A549CisR的EMT表型和迁移潜力,使其达到与母细胞A549相同的水平,增加E-Cadherin表达,随后降低ABCC1和ALDH3A1的表达。我们的数据表明,ALDH抑制剂DSF和ITC是顺铂化疗的潜在辅助治疗药物。DOI:10.3390/ijms23158644
文献信息
-
T3P® – A Benign Desulfurating Reagent in the Synthesis of Isothiocyanates作者:Tadeusz Gajda、Łukasz Janczewski、Anna Gajda、Sebastian Frankowski、Tomasz GoszczyńskiDOI:10.1055/s-0036-1591842日期:2018.3Abstract A number of alkyl, aryl and bifunctional isothiocyanates are obtained in moderate to high yields (41–94%) in a two-step, one-pot reaction of the parent primary amines or their salts with carbon disulfide, followed by reaction of the thus formed dithiocarbamates with T3P® (propane phosphonic acid anhydride) as a new and efficient desulfurating agent. A number of alkyl, aryl and bifunctional isothiocyanates
-
Direct, Microwave-Assisted Synthesis of Isothiocyanates作者:Łukasz Janczewski、Anna Gajda、Tadeusz GajdaDOI:10.1002/ejoc.201900105日期:2019.4.16Application of microwave technique allowed to accomplish novel, general and “greener” one‐pot protocol for the synthesis of isothiocyanates from amines. Reactions are easily scalable and take place without racemization of chiral amines. Decomposition of the intermediate dithiocarbamates into isothiocyanates proceeds without any additional desulfurating agent under these conditions.
-
A new diphenylphosphinite ionic liquid (IL-OPPh2) as reagent and solvent for highly selective bromination, thiocyanation or isothiocyanation of alcohols and trimethylsilyl and tetrahydropyranyl ethers作者:Nasser Iranpoor、Habib Firouzabadi、Roya AzadiDOI:10.1016/j.tetlet.2006.05.145日期:2006.7A new diphenylphosphinite ionic liquid (IL-OPPh2) is introduced. This ionic liquid is used as both a reagent and a solvent to convert alcohols and trimethylsilyl and tetrahydropyranyl (THP) ethers into their corresponding alkyl bromides, thiocyanates or isothiocyanates in the presence of Br2 and SCN− at 80 °C. In this ionic liquid, bromination and thiocyanation of alcohols occurs highly selectively
-
Chlorodiphenylphosphine as Highly Selective and Efficient Reagent for the Conversion of Alcohols, Tetrahydropyranyl and Silyl Ethers to Thiocyanates and Isothiocyanates作者:Ghasem Aghapour、Ameneh AsgharzadehDOI:10.1080/10426507.2013.855771日期:2014.6.3efficient method is described for the conversion of primary alcohols, tetrahydropyranyl and silyl ethers to thiocyanates by use of chlorodiphenylphosphine and ammonium thiocyanate. Secondary substrates produce both the two isomeric products, thiocyanate and isothiocyanate, while tertiary ones give isothiocyanates as the only products by this new method. In contrast to previously reported methods based
-
Preparation of thiocyanates and isothiocyanates from alcohols, thiols, trimethylsilyl-, and tetrahydropyranyl ethers using triphenylphosphine/2,3-dichloro-5,6-dicyanobenzoquinone (DDQ)/n-Bu4NSCN system作者:Nasser Iranpoor、Habib Firouzabadi、Najmeh NowrouziDOI:10.1016/j.tet.2006.03.030日期:2006.6A combination of triphenylphosphine (PPh3) and 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) provides a safe and easily available mixed reagent system for the conversion of 1° and 2° alcohols, thiols, trimethylsilyl-, and tetrahydropyranyl ethers to their corresponding thiocyanates and the 3° ones to isothiocyanates in good to high yields.
表征谱图
-
氢谱1HNMR
-
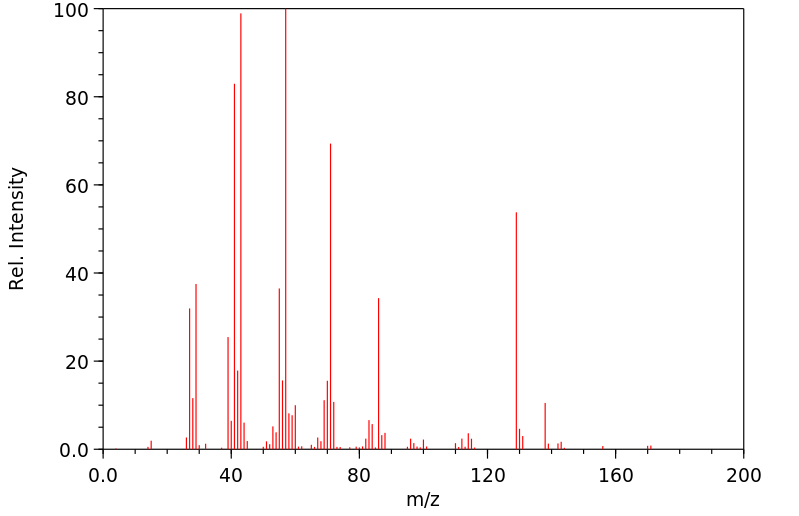
质谱MS
-
碳谱13CNMR
-
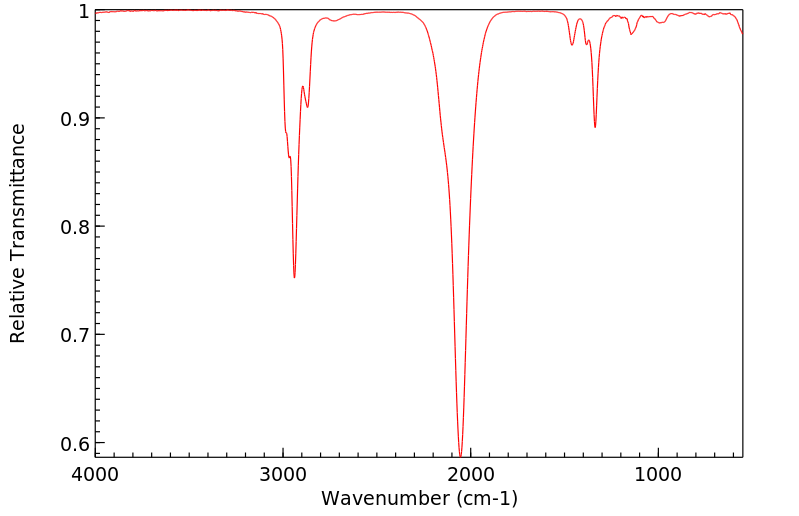
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-异硫氰基-1-环戊羧酸乙酯
顺-2-异硫氰基-1-环己烷羧酸乙酯
羰基异氰酸酯异硫氰酸酯
羰基二异硫氰酸酯
硫代异氰酸环戊酯
甲代烯丙基异硫氰酸酯
环己烷羰基异硫氰酸酯
环己基异硫氰酸脂
环丙基甲基异硫氰酸酯
环丁烷羰基异硫氰酸酯
异硫氰酸甲酯
异硫氰酸甲氧基甲酯
异硫氰酸甲基环己酯
异硫氰酸环辛酯
异硫氰酸环庚脂
异硫氰酸环十二酯
异硫氰酸环丙酯
异硫氰酸烯丙酯
异硫氰酸溴代乙酯
异硫氰酸氯代乙酯
异硫氰酸氨基甲酰
异硫氰酸异戊酯
异硫氰酸异丙酯
异硫氰酸异丁酯
异硫氰酸己酯
异硫氰酸叔辛酯
异硫氰酸十六酯
异硫氰酸十一烷酯
异硫氰酸仲丁酯
异硫氰酸乙酰酯
异硫氰酸乙酯
异硫氰酸乙烯
异硫氰酸3-丁烯酯
异硫氰酸2-甲氧基乙酯
异硫氰酸1-金刚烷酯
异硫氰酰甲酸甲酯
异硫氰酰甲酸乙酯
异硫氰基环丁烷
异硫代氰酰基乙醛二甲基乙缩醛
异氰酸丙酯
己烷,1-[(2-异硫氰基乙基)硫代]-
己烯,1-异硫氰基-
天然芥菜籽油
叔戊基异硫氰酸酯
叔丁基异硫氰酸酯
双(1-异硫氰基乙基)醚
十八烷基异氰酸酯
二(异硫氰酰甲基)醚
丙脒,N-丁基-2-氯-
三芥子酸甘油酯