2-氯嘌呤 | 1681-15-8
中文名称
2-氯嘌呤
中文别名
一缩二乙二醇一(2-乙基已基)醚;二甘醇(2-乙己基)醚;2-乙己基卡必醇;二甘醇一(2-乙己基)醚;二乙二醇单辛醚;一缩二乙二醇单(2-乙基已基)醚;2-{(2-[(2-乙基己基)氧基]乙氧基}乙醇;二甘醇单(2-乙己基)醚
英文名称
2-chloro-9H-purine
英文别名
2-Chlor-purin;2-chloropurine;2-chloro-7H-purine
CAS
1681-15-8
化学式
C5H3ClN4
mdl
MFCD00137826
分子量
154.559
InChiKey
JBMBVWROWJGFMG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:231-234 °C
-
沸点:287.9±23.0 °C(Predicted)
-
密度:1.84±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:10
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:54.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2933990090
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:2-8°C
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2-Chloro-7h-purine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Chloro-7h-purine
CAS number: 1681-15-8
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C5H3ClN4
Molecular weight: 154.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2-Chloro-7h-purine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Chloro-7h-purine
CAS number: 1681-15-8
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C5H3ClN4
Molecular weight: 154.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,8-二氯-9h-嘌呤 2,8-Dichlor purin 89166-91-6 C5H2Cl2N4 189.004 2,6-二氯嘌呤 2,6 dichloropurine 5451-40-1 C5H2Cl2N4 189.004 —— 2-chloro-1,7-dihydro-purine-6-thione 40769-62-8 C5H3ClN4S 186.625 2-氯-6-肼基-9h-嘌呤 6-chloro-2-hydrazinopurine 5404-88-6 C5H5ClN6 184.588 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-氯-9-甲基-9H-嘌呤 2-Chlor-9-methyl-purin 2346-73-8 C6H5ClN4 168.585 2-碘嘌呤 2-iodopurine 28128-16-7 C5H3IN4 246.01 2-溴-7H-嘌呤 2-bromopurine 28128-15-6 C5H3BrN4 199.01
反应信息
-
作为反应物:描述:参考文献:名称:The preparation and 1H n.m.r. spectra of some N-methylpurines and related compounds摘要:一些甲氧基、甲硫基和氯-N-甲基嘌呤及 对一些甲氧基、甲硫基和氯-N-甲基嘌呤以及咪唑并[4,5-c]-吡啶的 1H nm r 研究发现,当 N-甲基出现在六元环上时,其信号的场强比出现在咪唑环上时低。 六元环上的 N-甲基所产生的信号场低于咪唑环上的 N-甲基所产生的信号场。2-、6-和 8-甲氧基嘌呤的甲基化 和 2-、6-和 8-甲氧基嘌呤与重氮甲烷的甲基化反应,以及氯-N-甲基嘌呤与甲氧基-N-甲基嘌呤的甲基化反应。 介绍。DOI:10.1071/ch9830633
-
作为产物:描述:参考文献:名称:Efficient synthesis of nebularine and vidarabine via dehydrazination of (hetero)aromatics catalyzed by CuSO4in water摘要:首次在催化量的CuSO4存在下实现了一步简单的脱氢反应。以CuSO4 (2 mol%)为催化剂,水为溶剂,脱氢产物获得了良好的产率(66-95%)。此外,成功合成了药物 nebularine 和 Vidarabine,并且 Vidarabine 可在0.923公斤规模生产,显示出良好的工业应用潜力。DOI:10.1039/c3gc41658e
-
作为试剂:描述:3-(3-benzyloxy-4-methoxy-benzyl)-8-isopropyl-hypoxanthine 、 三氯氧磷 、 氨 、 盐酸 在 甲苯 、 2-氯嘌呤 、 四氢呋喃 、 crude product 作用下, 以 甲醇 为溶剂, -30.0~70.0 ℃ 、312.54 MPa 条件下, 反应 3.58h, 以to give 6-amino-3-(3-benzyloxy-4-methoxy-benzyl)-8-isopropyl-3H-purine hydrochloride (4.04 g, 91.8%)的产率得到3-[(4-methoxy-3-phenylmethoxyphenyl)methyl]-8-propan-2-ylpurin-6-amine;hydrochloride参考文献:名称:Purine derivatives having phosphodiesterase IV inhibition activity摘要:公开的是式(I)的化合物:其中,R3选自C1-10烷基,C1-10烯基,C3-10环烷基,C4-10环烷基烷基或C3-10环烯基的群,其中,所述烷基,烯基,环烷基,环烷基烷基或环烯基在一个位置上可以用羟基取代;或苄基,其中,所述苄基在一个或两个位置上可以用卤素,烷氧基,环烷氧基或多环烷基取代,其中,所述烷氧基或环烷氧基的烷基部分在一个位置上可以用羟基取代;R8选自氢,C1-10烷基,C1-10烯基,C3-10环烷基,C4-10环烷基烷基或C3-10环烯基的群,其中,所述烷基,烯基,环烷基,环烷基烷基或环烯基在一个位置上可以用羟基取代;或苄基,其中,所述苄基在一个或两个位置上可以用卤素,烷氧基,环烷氧基或多环烷基取代,其中,所述烷氧基或环烷氧基的烷基部分在一个位置上可以用羟基取代;R6a和R6b独立地选自氢,C1-10烷基,C1-10烯基,C3-10环烷基,C4-10环烷基烷基或C3-10环烯基的群,其中,所述烷基,烯基,环烷基,环烷基烷基或环烯基在一个位置上可以用羟基取代;以及其药学上可接受的盐。公开号:US06228859B1
文献信息
-
[EN] TRICYCLIC PI3K INHIBITOR COMPOUNDS AND METHODS OF USE<br/>[FR] COMPOSÉS TRICYCLIQUES INHIBITEURS DE PI3K ET PROCÉDÉS D'UTILISATION申请人:HOFFMANN LA ROCHE公开号:WO2012082997A1公开(公告)日:2012-06-21Tricyclic PI3k inhibitor compounds of Formula I with anti-cancer activity, anti- inflammatory activity, or immunoregulatory properties, and more specifically with PI3 kinase modulating or inhibitory activity are described. Methods are described for using the tricyclic PI3K inhibitor compounds of Formula I for in vitro, in situ, and in vivo diagnosis or treatment of mammalian cells, organisms, or associated pathological conditions. Formula I compounds include stereoisomers, geometric isomers, tautomers, and pharmaceutically acceptable salts thereof. The dashed lines indicate an optional double bond, and at least one dashed line is a double bond. The substituents are as described.
-
N-substituted nonaryl-heterocyclic NMDA/NR2B antagonists
-
Visible Light-Promoted Aliphatic C–H Arylation Using Selectfluor as a Hydrogen Atom Transfer Reagent作者:Hong Zhao、Jian JinDOI:10.1021/acs.orglett.9b01635日期:2019.8.16A mild, practical method for direct arylation of unactivated C(sp3)–H bonds with heteroarenes has been achieved via photochemistry. Selectfluor is used as a hydrogen atom transfer reagent under visible light irradiation. A diverse range of chemical feedstocks, such as alkanes, ketones, esters, and ethers, and complex molecules readily undergo intermolecular C(sp3)–C(sp2) bond formation. Moreover, a
-
[EN] PURINE DERIVATIVES USEFUL AS PI3 KINASE INHIBITORS<br/>[FR] DÉRIVÉS DE PURINE UTILES COMME INHIBITEURS DE PI3 KINASE申请人:HOFFMANN LA ROCHE公开号:WO2009053716A1公开(公告)日:2009-04-30This invention provides a compound which is a purine of formula (Ia) or (Ib): and the pharmaceutically acceptable salts thereof that are inhibitors of PI3K and a selective for the p110δ isoform, which is a class Ia PI3 kinase, over other class Ia PI3 kinases and over class Ib kinases. The compounds may be used to treat diseases and disorders arisi from abnormal cell growth, function or behaviour associated with PI3 kinase such as cance immune disorders, cardiovascular disease, viral infection, inflammation, metabolism/endocrine function disorders and neurological disorders.
-
[EN] PYRAZOLYLAMINOPURINES AS ITK INHIBITORS<br/>[FR] PYRAZOLYLAMINOPURINES EN TANT QU'INHIBITEURS DE LA TYROSYNE KINASE (ITK)申请人:HOFFMANN LA ROCHE公开号:WO2016091916A1公开(公告)日:2016-06-16Provided are pyrazolylaminopurine compounds that are inhibitors of ITK kinase, compositions containing these compounds and methods for treating diseases mediated by ITK kinase. In particular, provided are compounds of Formula I or II, stereoisomers, tautomers, solvates, prodrugs or pharmaceutically acceptable salts thereof, where n, R1, R2, R3, R4, R5, R6 and R7 are defined herein, pharmaceutical compositions comprising the compound and a pharmaceutically acceptable carrier, methods of using the compound or composition in therapy, for example, for treating a disease or condition mediated by ITK kinase in a patient.
表征谱图
-
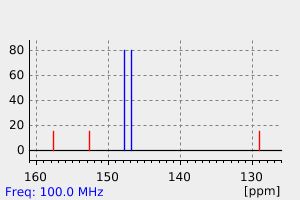
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
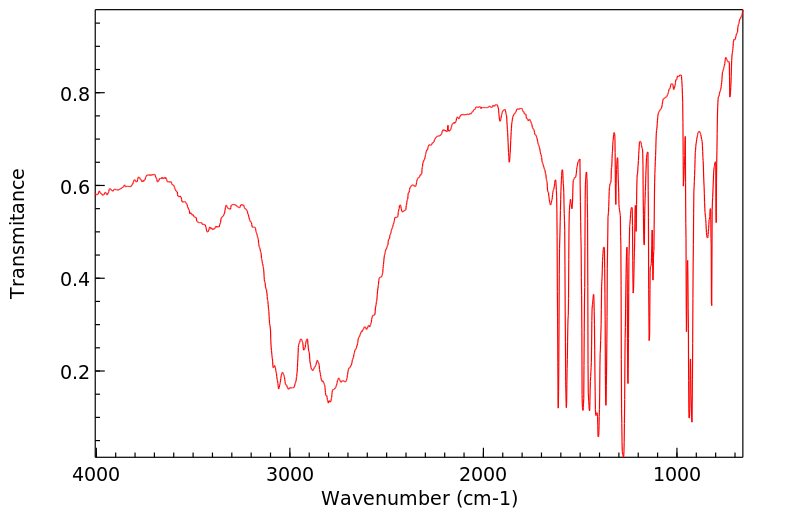
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄嘌呤钠盐
黄嘌呤
鸟嘌呤肟
鸟嘌呤盐酸盐
鸟嘌呤
顺式-二氨基二(O(6),9-二甲基鸟嘌呤-7)铂(II)二氯化物
顺式-2-(6-氨基-9H-嘌呤-9-基)-环己醇
阿罗茶碱
阿比茶碱
阿普西特-N-氧化物
阿昔洛韦钠
阿昔洛韦杂质K
阿昔洛韦杂质H
阿昔洛韦单磷酸盐
阿昔洛韦三磷酸酯
阿昔洛韦
阿德福韦酯杂质E
阿德福韦酯杂质12
阿德福韦酯杂质12
阿德福韦酯N6羟甲基杂质
阿德福韦酯 杂质C (阿德福韦单乙酯、单特戊酸甲酯)
阿德福韦酯
阿德福韦单特戊酸甲酯
阿德福韦-d4二磷酸三乙胺盐
阿德福韦
阿帕茶碱
阿司匹林,非那西汀和咖啡因
野杆菌素84
西潘茶碱
螺菲林
茶麻黄碱
茶苯海明
茶碱乙酸
茶碱一水合物
茶碱-D6
茶碱-8-丁酸
茶碱-2-氨基乙醇
茶碱
茶丙洛尔
苯酰胺,N-[9-[(2R)-2-羟基丙基]-9H-嘌呤-6-基]-
苯酰胺,N-(三甲基甲硅烷基)-N-[7-(三甲基甲硅烷基)-7H-嘌呤-6-基]-
苯酚,2-(3,4-二氢-2H-1-苯并吡喃-2-基)-
苯磺酸,4-(2,3,6,7-四氢-1,3,7-三甲基-2,6-二羰基-1H-嘌呤-8-基)-
苯甲酸咖啡鹼
苯甲腈,4-[(6,7-二氢-6-羰基-3H-嘌呤-3-基)甲基]-
苯呤司特
苄吡喃腺嘌呤
芬乙茶碱
芬乙茶碱
艾米替诺福韦