2-苄基-1-硝基胍 | 5415-72-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:183.5 °C(Solv: ethanol (64-17-5))
-
沸点:327.2±35.0 °C(Predicted)
-
密度:1.34±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.9
-
重原子数:14
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:96.2
-
氢给体数:2
-
氢受体数:3
安全信息
-
海关编码:2925290090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Sirakawa, Yakugaku Zasshi/Journal of the Pharmaceutical Society of Japan, 1959, vol. 79, p. 1487,1491摘要:DOI:
-
作为产物:描述:参考文献:名称:Davis; Luce, Journal of the American Chemical Society, 1927, vol. 49, p. 2304摘要:DOI:
文献信息
-
Facile preparation of nitroguanidines containing a sulfoximine motif作者:Qun Shao、Hong-sen Li、Yu-long Xiao、Ya Li、Lin-jing Zhao、Xin-feng Ren、Yuan Liu、Jia-qing LiuDOI:10.1016/j.tetlet.2019.151450日期:2020.1synthesis of nitroguanidines containing a sulfoximine motif has been developed. A two-step process starts with the oxidation coupling of sulfides and N-alkyl nitroguanidines to form sulfanylidenes, followed by oxidation with m-chloroperbenzoic (m-CPBA) to give the target sulfoximine-nitroguanidines. The reaction had a broad substrate scope with respect to both sulfides and N-alkyl nitroguanidines under mild
-
Reaction of Aromatic Heterocyclic Nitro Compounds with Potassium Cyanide. III. Some Nitroquinoline 1-Oxides作者:TOSHIHIKO OKAMOTO、HIROSHI TAKAHASHIDOI:10.1248/cpb.19.1809日期:——The reaction of 3-nitroquinoline 1-oxide with potassium cyanide in methanol was carried out producing 3-methoxycinchoninonitrile 1-oxide (I) and 3-methoxy-1H-pyrazolo [4, 3-b] quinoline-9-carboxamide (II). 5-Nitroquinoline 1-oxide gave 5-methoxyquinoline-6-carbonitrile 1-oxide (XIII) and 7-aminoisoxazolo [3, 4-f] quinoline 1-oxide (XIV), whereas 6-nitroquinoline 1-oxide gave methyl 5-cyano-6-methoxyquinaldimidate 1-oxide (VI), 5-cyano-6-methoxyquinaldamide 1-oxide (VII), and 1-hydroxy-6-nitrocarbostyril (VIII) by the similar type reactions. 4-Nitroquinoline 1-oxide produced 4-methoxyquinoline 1-oxide.
-
Design, Synthesis, and Antifungal Activity of Sulfoximine Derivatives Containing Nitroguanidine Moieties作者:Yulong Xiao、Hongsen Li、Qun Shao、Yuan Liu、Yonghai Xie、Linjing Zhao、Ya LiDOI:10.1002/cbdv.202100839日期:2022.3discover novel pesticide candidates, a series of sulfoximine derivatives were designed and synthesized via the oxidation coupling reaction of sulfides and N-alkyl nitroguanidines. The compounds were evaluated for their antifungal activity against six phytopathogenic fungi. Most of them exhibited a broad spectrum of fungicidal activity in vitro. Compound 8IV-b displayed good fungicidal activity against为了发现新的候选农药,通过硫化物和N-烷基硝基胍的氧化偶联反应设计和合成了一系列亚砜亚胺衍生物。评估了这些化合物对六种植物病原真菌的抗真菌活性。它们中的大多数在体外表现出广谱的杀菌活性。化合物8IV-b对核盘菌、立枯丝核菌、灰霉病菌、禾谷镰刀菌和辣椒疫霉表现出良好的杀菌活性,EC 50值分别为 12.82、12.50、17.25、31.08 和 30.11 mg/L。此外,化合物8III-c和8IV-e对辣椒红的EC 50值分别为22.23和20.67 mg/L ,明显优于市售腐霉利(118.15 mg/L) 。引人注目的是,8IV-d对B. cinerea表现出令人满意的杀菌活性,其 EC 50值(7.42对10.83 mg/L)与对照腐霉利相当,体内生物测定进一步证实8IV-d具有有效的保护作用对B. cinerea的影响200 毫克/升 (72.2 %)。目前的这些发现将促进新型强效杀菌剂的设计和开发。
-
THE ACIDITY OF NITROGUANIDINE AND ITS HOMOLOGUES作者:A. A. Amos、P. D. Cooper、E. Nishizawa、George F WrightDOI:10.1139/v61-235日期:1961.9.1
It has been shown that nitroguanidine, nitriminoimidazolidine, and 1-benzyl-2-nitriminoimidazolidine behave like urea by formation of potassium salts which completely hydrolyze in water. By contrast to true primary nitramines with which they might be considered to be tautomeric, the nitrimines are classed like urea as practically neutral compounds. Despite this ostensible tautomerism which was formerly evoked to explain the survival of nitroguanidine and nitriminoimidazolidine in alkaline solution it is now believed that the salts of nitroguanidine and nitraminoimidazolidine, from which the original nitrimines are regenerated upon acidification, are not intermediates, but rather end products, in the slow reaction with alkali. The intermediate is now postulated as an hydroxyisonitraminate anion from which the several products of the reaction are formed by appropriate fission. Presumably this anionic intermediate also is operative when tetraethylnitroguanidine or 1,3-dibenzyl-2-nitriminoimidazolidine is converted by alkali into tetraethylurea and 1,3-dibenzyl-2-inuclazolidine respectively.
已经显示,硝基胍、硝基亚甲基咪唑烷和1-苄基-2-硝基亚甲基咪唑烷通过形成钾盐的方式表现出类似尿素的行为,这些盐在水中完全水解。与真正的原生硝基胺相比,它们可能被认为是互变异构体,硝基亚胺类似于尿素,被分类为几乎是中性的化合物。尽管这种表面上的互变异构性曾被用来解释硝基胍和硝基亚甲基咪唑烷在碱性溶液中的存活,但现在人们认为硝基胍和硝基亚甲基咪唑烷的盐在酸化后重新生成原始硝基亚胺,不是中间体,而是与碱反应缓慢的终产物。现在假设中间体是一个羟基异硝酸盐阴离子,通过适当的裂解形成反应的几种产物。据推测,当四乙基硝基胍或1,3-二苄基-2-硝基亚甲基咪唑烷通过碱转化为四乙基尿素和1,3-二苄基-2-咪唑烷时,这种阴离子中间体也起作用。 -
一种硝基胍类化合物及其制备与应用申请人:上海工程技术大学公开号:CN110903227B公开(公告)日:2021-09-10
表征谱图
-
氢谱1HNMR
-
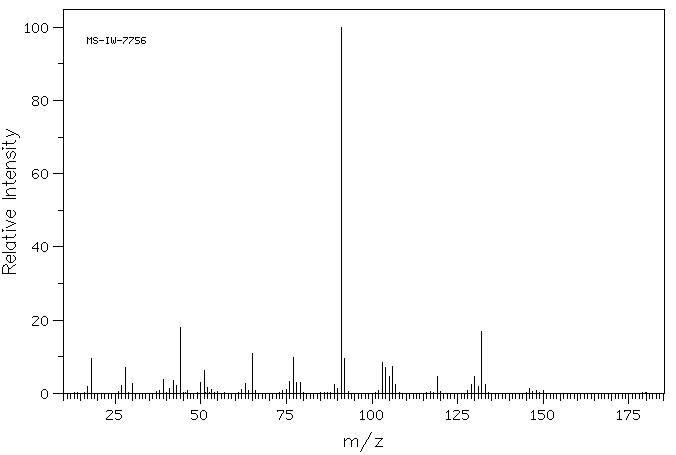
质谱MS
-
碳谱13CNMR
-
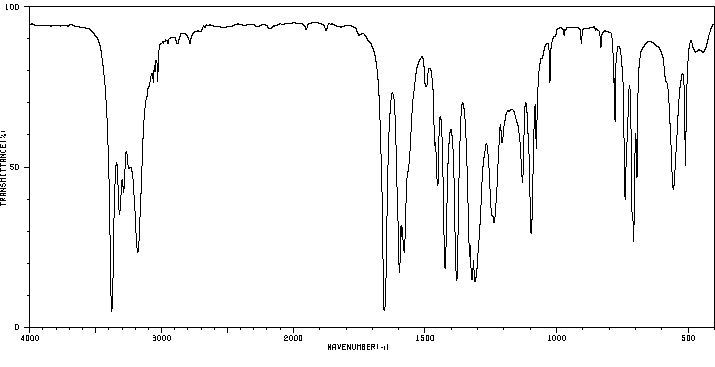
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息