(E)-2-(benzoyloxy)-4-phenylbut-3-enenitrile | 108583-47-7
中文名称
——
中文别名
——
英文名称
(E)-2-(benzoyloxy)-4-phenylbut-3-enenitrile
英文别名
2-benzoyloxy-4-phenyl-but-3-enenitrile;2-Benzoyloxy-4-phenyl-but-3-ennitril;α-Benzoyloxy-β-benzal-propionsaeure-nitril;α-Benzoyloxy-α-styryl-essigsaeurenitril;Zimtaldehyd-cyanhydrin-benzoat;3-Butenenitrile, 2-(benzoyloxy)-4-phenyl-;[(E)-1-cyano-3-phenylprop-2-enyl] benzoate
CAS
108583-47-7
化学式
C17H13NO2
mdl
——
分子量
263.296
InChiKey
HEHFCYOFVZHSGV-VAWYXSNFSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:64-65 °C
-
沸点:470.9±45.0 °C(Predicted)
-
密度:1.180±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:20
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.06
-
拓扑面积:50.1
-
氢给体数:0
-
氢受体数:3
反应信息
-
作为反应物:描述:(E)-2-(benzoyloxy)-4-phenylbut-3-enenitrile 、 3-溴丙烯 在 锌 作用下, 以 四氢呋喃 为溶剂, 反应 0.25h, 以100%的产率得到(E)-N-(4-allyl-3-hydroxy-1-phenylhepta-1,6-dien-4-yl)benzamide参考文献:名称:锌介导的功能性腈的双加成摘要:摘要 烯丙基锌试剂用于从氰基酸酯和氰基碳酸酯以高收率获得高度官能化的叔卡宾胺衍生物。虽然通常观察到有机金属在腈上的单加成,但在这项工作中,由于羰基部分中间转移到氮原子上,亲核烯丙基化发生了两次。反应的化学选择性允许存在各种官能团,在碳酸盐衍生物的情况下,通过动力学控制来调节最终产物的性质,从而选择性地得到羟酰胺或环状氨基甲酸酯。 烯丙基锌试剂用于从氰基酸酯和氰基碳酸酯以高收率获得高度官能化的叔卡宾胺衍生物。虽然通常观察到有机金属在腈上的单加成,但在这项工作中,由于羰基部分中间转移到氮原子上,亲核烯丙基化发生了两次。反应的化学选择性允许存在各种官能团,在碳酸盐衍生物的情况下,通过动力学控制来调节最终产物的性质,从而选择性地得到羟酰胺或环状氨基甲酸酯。DOI:10.1055/s-0037-1611704
-
作为产物:描述:参考文献:名称:三乙胺催化无溶剂合成氰醇衍生物摘要:已经优化了一种非常简单的一步环保程序,用于在没有溶剂的情况下,使用最少量的相应氰化物,从醛和酮合成 0-取代的氰醇。使用三乙胺作为催化剂,醛比酮反应更快,在这两种情况下都提供了相应的 O-三甲基甲硅烷基、O-甲氧基羰基、O-苯甲酰基和 O-乙酰羟腈的几乎定量产率。DOI:10.1055/s-2005-872096
文献信息
-
A Simple One-Pot Synthesis of Silylated and Acylated Cyanohydrins作者:Ryuji Yoneda、Kazunori Santo、Shinya Harusawa、Takushi KuriharaDOI:10.1055/s-1986-31873日期:——Reaction of variety of aldehydes and ketones with chlorotrimethylsilane and acyl chlorides in the presence of lithium cyanide to give trimethylsilylcyanohydrins and acylcyanohydrins is described.
-
Francis; Davis, Journal of the Chemical Society, 1909, vol. 95, p. 1406作者:Francis、DavisDOI:——日期:——
-
Aloy; Rabaut, Bulletin de la Societe Chimique de France, 1918, vol. <4> 23, p. 99作者:Aloy、RabautDOI:——日期:——
-
YONEDA RYUJI; SANTO KAZUNORI; HARUSAWA SHINYA; KURIHARA TAKUSHI, SYNTHESIS,(1986) N 12, 1054-1055作者:YONEDA RYUJI、 SANTO KAZUNORI、 HARUSAWA SHINYA、 KURIHARA TAKUSHIDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
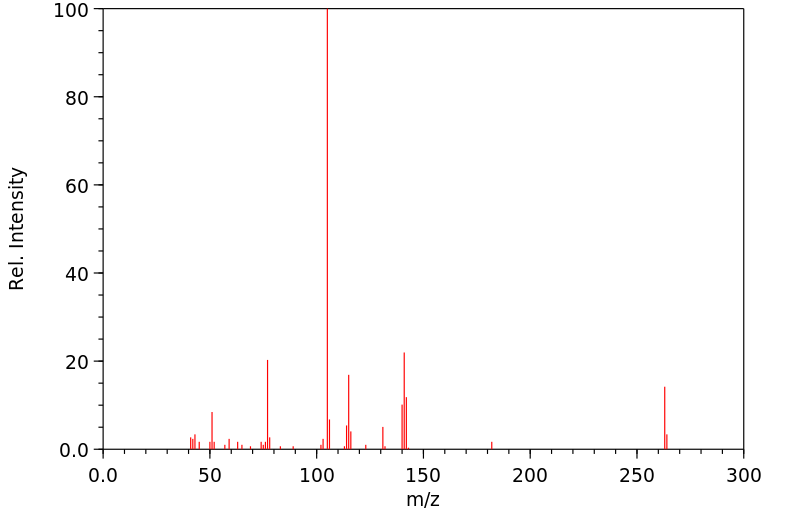
质谱MS
-
碳谱13CNMR
-
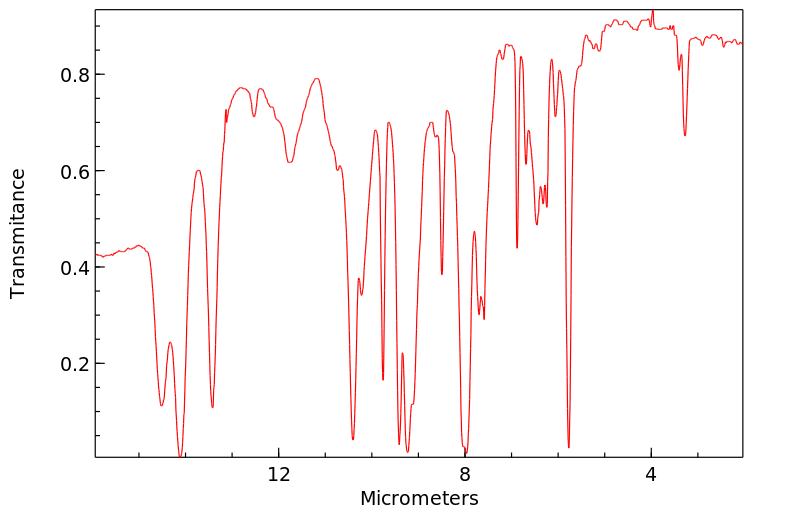
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫