3,4-二甲基苯硼酸频呐醇酯 | 401797-00-0
中文名称
3,4-二甲基苯硼酸频呐醇酯
中文别名
3,4-二甲基苯硼酸频那醇酯
英文名称
2-(3,4-dimethylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
英文别名
4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-o-xylene;3,4-dimethylphenylboronic acid pinacol ester;1,2-dimethyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzene
CAS
401797-00-0
化学式
C14H21BO2
mdl
——
分子量
232.131
InChiKey
HWIMTTLCFONAAB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:35 °C
-
沸点:325.4±31.0 °C(Predicted)
-
密度:0.97±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:17
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.57
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2934999090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:0-10°C
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 3,4-Dimethylphenylboronic acid pinacol ester
Synonyms: 2-(3,4-Dimethylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 3,4-Dimethylphenylboronic acid pinacol ester
CAS number: 401797-00-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C14H21BO2
Molecular weight: 232.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 3,4-Dimethylphenylboronic acid pinacol ester
Synonyms: 2-(3,4-Dimethylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 3,4-Dimethylphenylboronic acid pinacol ester
CAS number: 401797-00-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C14H21BO2
Molecular weight: 232.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:参考文献:名称:铜促进硼酯和二氮杂环丁酮的 C-N 偶联:一种制备芳基脲的方法摘要:描述了一种新的铜促进硼酯和二叔丁基二氮杂环丁酮之间的 C-N 偶联。在温和条件下可以很容易地获得多种芳基脲,产率高达 92%。DOI:10.1021/acs.orglett.1c03468
-
作为产物:描述:参考文献:名称:钯催化的甲磺酸烷基酯和甲苯磺酸酯的硼化反应及其在一锅顺序铃木-宫浦联芳的合成中的应用摘要:一锅之巅!描述了芳基甲苯磺酸盐和甲磺酸盐的第一个钯催化的硼化反应。反应条件温和,并具有出色的官能团相容性(例如,R = CN,CHO,COOMe,C(O)R,NH 2或NH-吲哚;请参见方案)。Pd / MeO-CM-phos可在不对称联芳烃的制备中进行一锅法依次反应。DOI:10.1002/chem.201100361
文献信息
-
<scp>Pd‐Catalyzed Site‐Selective</scp> Borylation of Simple Arenes <i>via</i> Thianthrenation <sup>†</sup>作者:Xiao‐Yue Chen、Yu‐Hao Huang、Jian Zhou、Peng WangDOI:10.1002/cjoc.202000212日期:2020.11Site‐selective borylation of simple arenes was realized in one pot via an electrophilic thianthrenation/Pd‐catalyzed borylation sequence. The key to achieve this operatically simple process is the use of Pd catalysis, which could tolerate the solvent and acidic conditions used in the thianthrenation step. This protocol features mild conditions, broad functional group tolerance, and simple manipulations
-
Single-Site Cobalt-Catalyst Ligated with Pyridylimine-Functionalized Metal–Organic Frameworks for Arene and Benzylic Borylation作者:Rajashree Newar、Wahida Begum、Neha Antil、Sakshi Shukla、Ajay Kumar、Naved Akhtar、Balendra、Kuntal MannaDOI:10.1021/acs.inorgchem.0c00747日期:2020.8.3also significantly more robust and active than its homogeneous control, highlighting the beneficial effect of active-site isolation within the MOF framework that prevents intermolecular decomposition. The experimental and computational studies suggested (pyrim•−)CoI(THF) as the active catalytic species within the MOF, which undergoes a mechanistic pathway of oxidative addition, turnover limiting σ-bond我们报告了一种基于多孔且坚固耐用的吡啶基嘧啶功能化的金属有机骨架(pyrim-MOF)的高活性单中心非均相钴催化剂,用于芳烃和苄基CH键的化学选择性硼化。由U-O-68拓扑结构构成的嘧啶-MOF由锆-簇的二级结构单元和嘧啶基官能化的二羧酸桥接桥连接体构成,先用CoCl 2进行金属化,然后用NaEt 3 BH处理,得到钴官能化的MOF催化剂(pyrim- MOF-Co)。Pyrim-MOF-Co具有广泛的底物范围,允许使用B 2销2对卤素,烷氧基,烷基取代的芳烃以及杂环系统进行C–H硼化或用HBpin(pin =频哪醇酯)作为硼酸酯化剂,以高收率得到相应的芳烃或烷基硼酸酯。Pyrim-MOF-Co的营业额(TON)高达2500,并且可以循环使用至少9次。Pyrim-MOF-Co还比其均质控件强得多,并且更具活性,突出了在MOF框架内进行主动位点隔离以防止分子间分解的有益作用。实验和计算研究表明(pyrim
-
Metal-organic frameworks containing nitrogen-donor ligands for efficient catalytic organic transformations申请人:The University of Chicago公开号:US10647733B2公开(公告)日:2020-05-12Metal-organic framework (MOFs) compositions based on nitrogen donor-based organic bridging ligands, including ligands based on 1,3-diketimine (NacNac), bipyridines and salicylaldimine, were synthesized and then post-synthetically metalated with metal precursors, such as complexes of first row transition metals. Metal complexes of the organic bridging ligands could also be directly incorporated into the MOFs. The MOFs provide a versatile family of recyclable and reusable single-site solid catalysts for catalyzing a variety of asymmetric organic transformations. The solid catalysts can also be integrated into a flow reactor or a supercritical fluid reactor.
-
Room temperature Pd(0)/Ad3P-catalyzed coupling reactions of aryl chlorides with bis(pinacolato)diboron作者:Jie Dong、Hui Guo、Wei Peng、Qiao-Sheng HuDOI:10.1016/j.tetlet.2019.01.042日期:2019.3Pd(0)/Ad3P-catalyzed cross-coupling reactions of aryl chlorides with bis(pinacolato)diboron are described. The Pd(0)/Ad3P catalyst, generated from Ad3P-coordinated acetanilide-based palladacycle complex, proved to be an efficient catalyst system for the Miyaura borylation reactions of a variety of aryl chlorides with bis(pinacolato)diboron. The mild reaction condition, the easy availability of the catalyst
-
Hydrogenation of (Hetero)aryl Boronate Esters with a Cyclic (Alkyl)(amino)carbene–Rhodium Complex: Direct Access to <i>cis</i> ‐Substituted Borylated Cycloalkanes and Saturated Heterocycles作者:Liang Ling、Yuan He、Xue Zhang、Meiming Luo、Xiaoming ZengDOI:10.1002/anie.201811210日期:2019.5.13We herein report the hydrogenation of substituted aryl‐ and heteroaryl boronate esters for the selective synthesis of cis‐substituted borylated cycloalkanes and saturated heterocycles. A cyclic (alkyl)(amino)carbene‐ligated rhodium complex with two dimethyl groups at the ortho‐alkyl scaffold of the carbene showed high reactivity in promoting the hydrogenation, thereby enabling the hydrogenation of
表征谱图
-
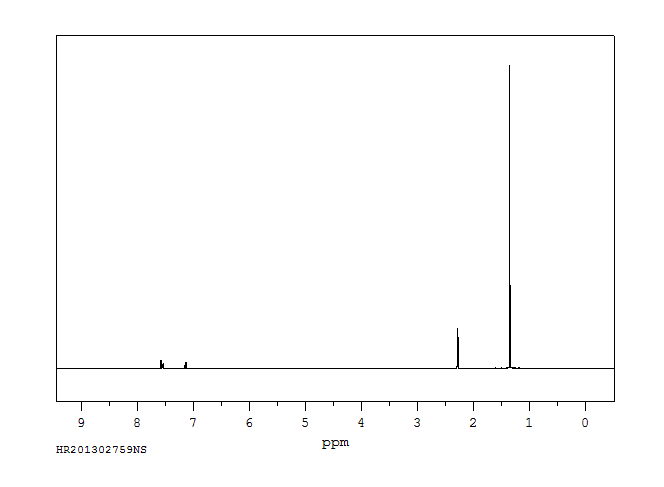
氢谱1HNMR
-
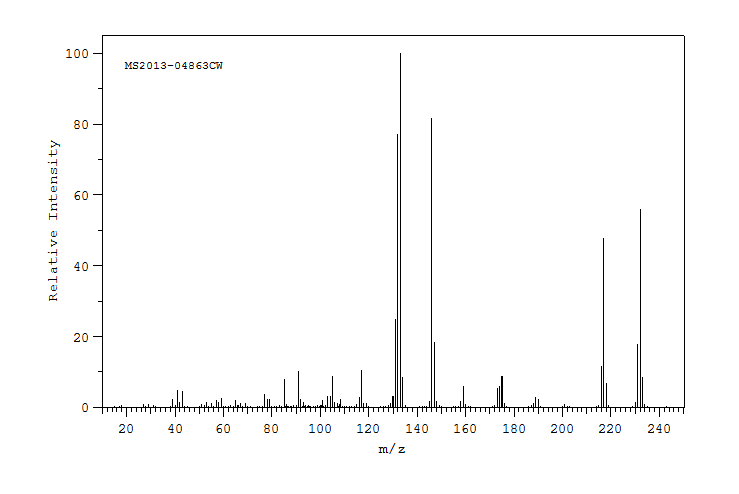
质谱MS
-
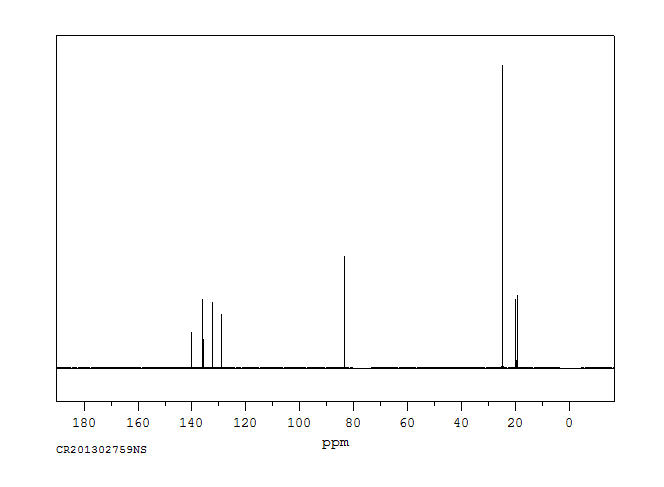
碳谱13CNMR
-
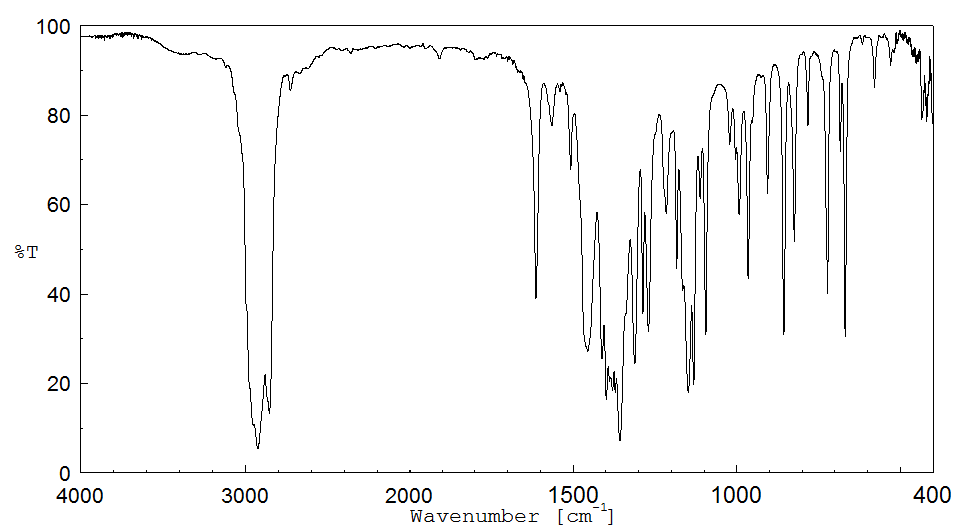
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫