3,4-双(三氟甲基)二苯甲酮 | 21084-22-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:95-97 °C(lit.)
-
沸点:326.4±42.0 °C(Predicted)
-
密度:1.3935 (estimate)
-
稳定性/保质期:
在常温常压下保持稳定,应避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):5
-
重原子数:22
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.133
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:7
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2914700090
-
安全说明:S26,S37/39
-
储存条件:将物品存放在阴凉、干燥的密闭容器中保存。
SDS
| Name: | 3 4 -Bis(Trifluoromethyl)benzophenone 98% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 21084-22-0 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 21084-22-0 | 3,4'-Bis(Trifluoromethyl)benzophenone | 98% | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
No information regarding eye irritation and other potential effects was found.
Skin:
No information regarding skin irritation and other potential effects was found.
Ingestion:
The toxicological properties of this substance have not been fully investigated. Exposure to fluoride compounds can result in systemic toxic effects on the heart, liver, and kidneys. It may also deplete calcium levels in the body leading to hypocalcemia and death.
Inhalation:
The toxicological properties of this substance have not been fully investigated.
Chronic:
Chronic inhalation and ingestion may cause chronic fluoride poisoning (fluorosis) characterized by weight loss, weakness, anemia, brittle bones, and stiff joints. Chronic exposure to fluoride compounds may cause systemic toxicity.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid immediately. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use only in a well-ventilated area.
Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 21084-22-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 95 - 97 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: (CF3C6H4)2CO
Molecular Weight: 318.22
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable.
Conditions to Avoid:
Not available.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, hydrogen fluoride gas.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 21084-22-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3,4'-Bis(Trifluoromethyl)benzophenone - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 21084-22-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 21084-22-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 21084-22-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:3,4-双(三氟甲基)二苯甲酮 、 肼甲酰亚胺酰胺一氯化氢 在 盐酸 作用下, 以 乙二醇 为溶剂, 生成 4,3'-Bis-(trifluormethyl)-benzophenon-guanylhydrazon参考文献:名称:芳族酮的guan盐的抗疟活性。I.主要寻找活性取代基图案。摘要:DOI:10.1021/jm00301a006
-
作为产物:描述:参考文献:名称:芳族酮的guan盐的抗疟活性。I.主要寻找活性取代基图案。摘要:DOI:10.1021/jm00301a006
文献信息
-
Formation of Bromocarbenium Bromide Ion Pairs in the Electrophilic Bromination of Highly Reactive Olefins in Chlorinated Aprotic Solvents作者:Giuseppe Bellucci、Cinzia Chiappe、Giacomo Lo MoroDOI:10.1021/jo9620526日期:1997.5.1rate-determining nucleophilic attack by bromide on the olefin-Br(2) pi-complex. The data related to the bromination of the more activated methoxystilbenes are rationalized considering that, for these olefins, even in aprotic solvents, the ionization of the initially formed 1:1 pi-complex to a bromocarbenium bromide ion pair can compete both with the formation of a bromonium-tribromide ion pair and with the在不同温度下,在非质子传递溶剂中研究了几种取代的丁苯醚与四丁基三溴化铵(TBAT)的动力学和溴化产物。带有吸电子或适度给电子取代基的对苯二酚立体定向地提供抗加成产物。反应遵循二级速率定律,并且发现顺-苯乙烯的溴化反应的逆动力学同位素效应(KIE)k(H)/ k(D)= 0.85(0.05)。顺式-和反式-4,4-二甲氧基苯乙烯的反应生成内消旋和d,l二溴化物在氯仿和1,2-二氯乙烷中的混合物。对于后一种烯烃测得的速率常数(k(Br)()3()-)与Hammett相关性有很大偏差,添加溴化物对速率有很大影响。这些活化的对苯二酚与分子Br(2)的反应在低Br(2)浓度下进行,遵循混合的二阶/三阶速率定律。带有吸电子或适度给电子取代基的斯蒂苯酯与TBAT反应的动力学和产物分布数据是根据已知的机理进行解释的,该机理涉及产物和速率决定了溴化物对烯烃的亲核进攻。 Br(2)pi络合物。考虑到对于这些烯
-
FUNCTIONALIZED PHOTOREACTIVE COMPOUNDS申请人:Bury Izabela公开号:US20100266821A1公开(公告)日:2010-10-21The present invention relates to a photoreactive compound for the preparation of a photoalignment material comprising thioether units, wherein the photoreactive compound is comprising at least one ene group and at least one photoalignment group, and further to a composition comprising at least one photoreactive compound and at least one polythiol, the use of this composition for the preparation of photoalignment materials, and their use for the alignment of liquid crystals or liquid crystal polymers, in electro-optical and optical elements, systems and devices.
-
Silver‐Catalyzed Dearomative Skeletal Editing of Indazoles by Donor Carbene Insertion作者:Linxuan Li、Hongzhu Chen、Menglin Liu、Qingwen Zhu、Hongru Zhang、Graham de Ruiter、Xihe BiDOI:10.1002/chem.202304227日期:2024.3.15
Abstract Given the prevalence of heterocyclic scaffolds in drug‐related molecules, converting these highly modular heterocyclic scaffolds into structural diversified and dearomatized analogs is an ideal strategy for improving their physicochemical and pharmacokinetic properties. Here, we described an efficient method for silver carbene‐mediated dearomative N−N bond cleavage leading to skeletal hopping between indazole and 1,2‐dihydroquinazoline via a highly selective single‐carbon insertion procedure. Using this methodology, a series of dihydroquinazoline analogues with diarylmethylene‐substituted quaternary carbon centers were constructed with excellent yields and good functional group compatibility, which was further illustrated by the late‐stage diversification of important pharmaceutically active ingredients. DFT calculations indicated that the silver catalyst not only induces the formation of the silver carbene, but also activates the diazahexatriene intermediate, which plays a crucial role in the formation of the C−N bond.
-
TETRAHYDROBENZINDOLE DERIVATIVES申请人:Meiji Seika Kaisha, Ltd.公开号:EP1057814A1公开(公告)日:2000-12-06This invention creates compounds represented by the following formula (1) which, alone or as a plurality of them simultaneously, act upon serotonin receptors, and thereby provides pharmaceutical compositions that contain these compounds and are useful for the treatment or prevention of diseases which are considered to be induced by the abnormality of central and peripheral serotonin controlling functions. In the formula, R1 is a hydrogen atom, a lower alkyl group or an aralkyl group; R2 is a hydrogen atom or a specified substituent; n is an integer of 2 to 6; and α is the following formula (a), (b), (c), (d) or (e). In formulae (a) and (b), R3 is a hydrogen atom or a specified substituent; X is NR10, NCONR11R12, S, SO, SO2 or O; R10 is a hydrogen atom or a specified substituent; R11 and R12 is independently a hydrogen atom or a lower alkyl; Y is a methylene group or a carbonyl group. In formula (c), R4 is a hydrogen atom or a specified substituent; R5 is a hydrogen atom or a specified substituent; k is 0 or an integer of 1 to 3; m is 0 or an integer of 1 to 3; each of A and B is a group which forms a specified ring via a double bond; k + m is an integer of 1 to 3. In formulae (d) and (e), R4 is as defined above; G is CH2, S, O or C=O; D is CH or N; p is an integer of 1 to 3; each of E and J is a group which forms a benzene ring or a pyridine ring via a double bond; R6 and R7 is independently a hydrogen atom or a lower alkyl or the like specified substituent.本发明创造了由下式(1)表示的化合物,这些化合物单独或多个同时作用于血清素受体,从而提供了含有这些化合物的药物组合物,这些组合物可用于治疗或预防被认为是由中枢和外周血清素控制功能异常诱发的疾病。 式中,R1 是氢原子、低级烷基或芳烷基;R2 是氢原子或特定取代基;n 是 2 至 6 的整数;α 是下式 (a)、(b)、(c)、(d) 或 (e)。 在式(a)和(b)中,R3 是氢原子或特定取代基;X 是 NR10、NCONR11R12、S、SO、SO2 或 O;R10 是氢原子或特定取代基;R11 和 R12 独立地是氢原子或低级烷基;Y 是亚甲基或羰基。在式(c)中,R4 是氢原子或特定取代基;R5 是氢原子或特定取代基;k 是 0 或 1 至 3 的整数;m 是 0 或 1 至 3 的整数;A 和 B 各自是通过双键形成特定环的基团;k + m 是 1 至 3 的整数。在式(d)和(e)中,R4 如上定义;G 是 CH2、S、O 或 C=O;D 是 CH 或 N;p 是 1 至 3 的整数;E 和 J 各自是通过双键形成苯环或吡啶环的基团;R6 和 R7 独立地是氢原子或低级烷基或类似的指定取代基。
-
Photoalignment composition申请人:ROLIC AG公开号:US10558089B2公开(公告)日:2020-02-11The present invention relates to a photoalignment composition comprising a) 0.001 to 20%, by weight; preferably, 1 to 10% by weight, more preferably 1 to 9% by weight of at least one photoreactive compound (I) that comprises a photoalignment group and b) 80 to 99.999% by weight, preferably, 90 to 99% by weight, more preferably 91 to 99% by weight of at least one compound (II) that does not comprise a photoalignment group, and c) optionally at least one reactive or non reactive additives, and d) optionally at least one solvent. Further, the present invention relates to the use of this photoalignment composition for the alignment of liquid crystals or liquid crystal polymers, in electro-optical and optical elements, systems and devices.本发明涉及一种光配位组合物,该组合物包含 a) 至少一种包含光配位基团的光活性化合物(I),其重量百分比为 0.001 至 20%;优选为 1 至 10%,更优选为 1 至 9%;以及 b) 至少一种不包含光配位基团的化合物(II),其重量百分比为 80 至 99.999%(重量),更优选90至99%(重量),更优选91至99%(重量)的至少一种不包含光配位基团的化合物(II),以及c)可选的至少一种活性或非活性添加剂,以及d)可选的至少一种溶剂。此外,本发明还涉及在电子光学和光学元件、系统和设备中将这种光配位组合物用于液晶或液晶聚合物的配位。
表征谱图
-
氢谱1HNMR
-
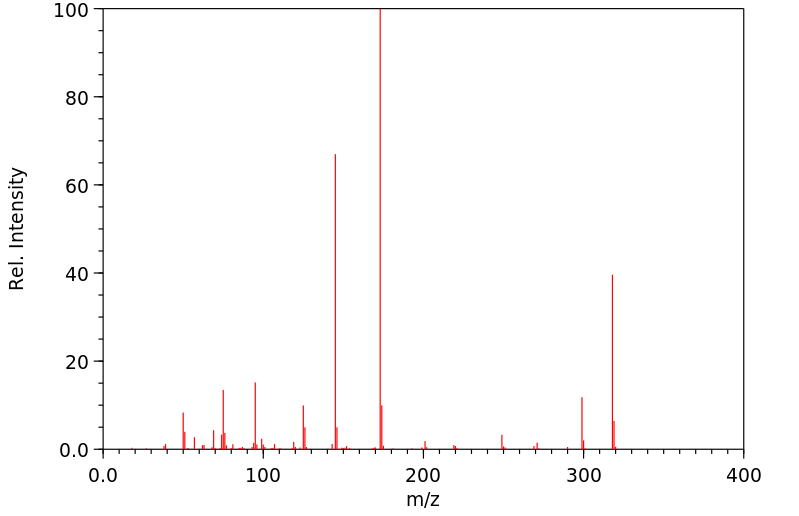
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息