(Z)-1-chloro-4-(prop-1-en-1-yloxy)benzene | 51896-45-8
中文名称
——
中文别名
——
英文名称
(Z)-1-chloro-4-(prop-1-en-1-yloxy)benzene
英文别名
Z-p-Chlorphenyl-1-propenylether;(4-Chlorophenyl) (1-propenyl) ether;1-chloro-4-[(Z)-prop-1-enoxy]benzene
CAS
51896-45-8
化学式
C9H9ClO
mdl
——
分子量
168.623
InChiKey
YQTLPMRVSPHBGA-UQCOIBPSSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:221.0±23.0 °C(Predicted)
-
密度:1.109±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.11
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Stereochemistry of electrophilic additions to linear enol ethers摘要:DOI:10.1021/jo00902a030
-
作为产物:描述:4-氯-烯丙氧基 在 (1R,2R)-(-)-N,N'-bis(3,5-di-tert-butylsalicydene)-1,2-cyclohexanediaminocobalt(II) 、 1,1,3,3-四甲基二硅氧烷 、 1-氟-2,4,6-三甲基吡啶三氟甲烷磺酸盐 作用下, 反应 5.0h, 以75%的产率得到(Z)-1-chloro-4-(prop-1-en-1-yloxy)benzene参考文献:名称:使用钴(II)(salen)配合物催化剂在烯丙基醚的氧化异构化中生成特定的Z-烯醇醚的Z选择性。摘要:烯醇醚的结构基序存在于许多高度氧化的生物活性天然产物和药物中。几何上不稳定的Z-烯醇醚的合成具有挑战性。已经开发出一种有效的Z-烯丙基醚(Zalen)配合物使用N-氟-2,4,6-三甲基吡啶三氟甲磺酸盐(Me3NFPY•OTf)作为氧化剂催化的烯丙基醚的Z选择氧化异构化方法。热力学稳定性较低的Z-烯醇醚以高收率和高几何控制制备。该方法还证明了在室温下控制二烯丙基醚的Z-选择性异构化反应的有效性。该催化体系提供了另一种途径来扩展烯丙基醚的传统还原异构化。DOI:10.1021/acs.joc.0c00004
文献信息
-
Thermodynamic, spectroscopic, and density functional theory studies of allyl aryl and prop-1-enyl aryl ethers. Part 1. Thermodynamic data of isomerization作者:Esko TaskinenDOI:10.1039/b101837j日期:——A chemical equilibration study of the relative thermodynamic stabilities of seventy isomeric allyl aryl ethers (a) and (Z)-prop-1-enyl aryl ethers (b) in DMSO solution has been carried out. From the variation of the equilibrium constant with temperature the Gibbs energies, enthalpies, and entropies of isomerization at 298.15 K have been evaluated. Because of their low enthalpies, the (Z)-prop-1-enyl aryl ethers are strongly favored at equilibrium, the Gibbs energies of the a→b isomerization ranging from −12 to −23 kJ mol−1. The entropy contribution is negligible in most reactions, but occasionally small positive values less than +10 J K−1 mol−1 of the entropy of isomerization are found. The equilibration studies were also extended to involve two pairs of related isomeric ethers with a Me substituent on C(2) of the olefinic bond. The Me substituent was found to increase the relative thermodynamic stability of the allylic ethers by ca. 3.4 kJ mol−1.
-
Ruthenium(IV)‐Catalyzed Isomerization of the CC Bond of<i>O</i>‐Allylic Substrates: A Theoretical and Experimental Study作者:Adrián Varela‐Álvarez、José A. Sordo、Estefanía Piedra、Noel Nebra、Victorio Cadierno、José GimenoDOI:10.1002/chem.201101131日期:2011.9.12carried out in aqueous medium. The new mechanistic proposal helped to develop a new experimental procedure for isomerization of allyl ethers to 1‐propenyl ethers under neutral aqueous conditions. This process is an unique example of efficient and selective catalytic isomerization of allyl ethers in aqueous medium.一般机制来合理化茹IV催化的所述CC键的异构化在ö建议使用烯丙基底物。在MPWB1K / 6-311 + G(d,p)+ SDD的理论水平上进行了支持该机制的计算。可以根据新机理解释在不同溶剂(水和THF)和不同pH条件(中性和碱性)下的所有实验观察结果。从预催化剂到催化剂的转化的理论分析导致了在不同介质中活性物种的结构鉴定。实验观察到的诱导期与为该过程计算的能垒的大小有关。如实验观察到的,催化循环的理论能量分布需要施加相对较高的温度。当在水性介质中进行反应时,水分子参与反应坐标是机械上必不可少的。新的机理建议有助于开发一种新的实验程序,用于在中性水溶液条件下将烯丙基醚异构化为1-丙烯基醚。该方法是烯丙基醚在水性介质中有效和选择性催化异构化的独特实例。
-
Lewis acid promoted double bond migration in O-allyl to Z-products by Ru-H complexes作者:Haibin Wang、Shaodong Liu、Tingting Sun、Zhanao Lv、Zhen Zhan、Guochuan Yin、Zhuqi ChenDOI:10.1016/j.mcat.2019.02.007日期:2019.5and convenient ruthenium(II) complex for the catalytic isomerization of O-allylethers, leading to thermodynamic-unfavored Z-product under mild conditions. The model substrate of allyl phenyl ether can be simply scaled up to 20 mmol to produce Z-product with TON of 2453 and TOF of 13,430 h−1 at 40–60 °C. The system of Ru(II)/Lewis Acid catalysts was suitable for various substituted O-allylethers and other在催化双键迁移反应中,通常以E-构型烯烃为主要产物,因为E-构型在热力学上是有利的。然而,由于结构-活性关系,有时在药物化学中需要Z-构型产物。在本文中,我们证明了一种新的策略,即路易斯酸促进了广泛使用且方便的钌(II)络合物用于O-烯丙基醚的催化异构化,导致在温和条件下产生热力学不利的Z产物。烯丙基苯基醚的模型底物可以简单地按比例放大至20 mmol以生产TON为2453,TOF为13,430 h -1的Z产物在40–60°C下。Ru(II)/路易斯酸催化剂体系适用于各种取代的O-烯丙基醚和其他类型的底物。通过包括动力学研究,配体抑制作用和分子光谱在内的机理研究,通过添加路易斯酸使PPh 3配体解离,以及由嵌合助剂形成五元Ru络合物均被认为是提高反应性和稳定性的必要步骤。通过金属氢化物加成消除机理控制催化双键迁移反应的立体选择性。这一新策略可能为生产用于药物化学的杂环化合物中热力学不利的产品提供新的机会。
-
Photochemical Radical Cation Cycloadditions of Aryl Vinyl Ethers作者:Sota Adachi、Genki Horiguchi、Hidehiro Kamiya、Yohei OkadaDOI:10.1002/ejoc.202201207日期:2022.11.25TiO2 photochemical [2+2] and [4+2] cycloadditions using aryl vinyl ethers as radical cation precursors. The construction of various four- and six-membered rings is made possible by these radical cation cycloadditions. The diene is found to be more radicalophilic than the alkene and effectively traps the radical cation of the aryl vinyl ethers.
表征谱图
-
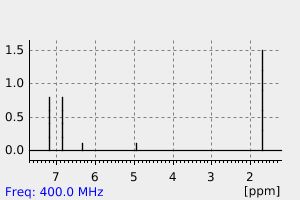
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫