3,5-二甲基-1,2,4-三硫环戊烷 | 23654-92-4
中文名称
3,5-二甲基-1,2,4-三硫环戊烷
中文别名
3,5-二甲基-1,2,4-三硫杂环戊烷;3,5-二甲基-1,2,4-三硫杂环己烷
英文名称
3,5-dimethyl-1,2,4-trithiolane
英文别名
3,5-Dimethyl-1,2,4-trithiolan;3,5-Dimethyl-<1,2,4>trithiolan
CAS
23654-92-4
化学式
C4H8S3
mdl
——
分子量
152.306
InChiKey
HFRUNLRFNNTTPQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:38 °C(Press: 0.3 Torr)
-
密度:1?+-.0.06 g/cm3(Predicted)
-
LogP:2.31
-
溶解度:insoluble in water; soluble in fat
-
折光率:1.593-1.603
-
保留指数:1189.1;1101;1100;1111;1097;1127;1133
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:75.9
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2934999090
SDS
制备方法与用途
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— methyl-1-[(RS)-1-((SR)-1-methylsulfanyl-ethylsulfanyl)-ethyl]-disulfane 69318-93-0 C6H14S4 214.441
反应信息
-
作为反应物:参考文献:名称:Bock, Hans; Hirabayashi, Takakuni; Mohmand, Shamsher, Chemische Berichte, 1982, vol. 115, # 2, p. 492 - 503摘要:DOI:
-
作为产物:描述:参考文献:名称:Asinger et al., Justus Liebigs Annalen der Chemie, 1959, vol. 627, p. 195,212摘要:DOI:
文献信息
-
A Novel Method for the Generation of Thial S-Sulfides from 2,4,6-Trisubstituted 5,6-Dihydro-1,3,5-dithiazines作者:Yuji Takikawa、Takahiro Makabe、Naoyuki Hirose、Takamichi Hiratsuka、Ryuji Takoh、Kazuaki ShimadaDOI:10.1246/cl.1988.1517日期:1988.9.5Treatment of 2,4,6-trisubstituted 5,6-dihydro-1,3,5-dithiazines with NCS or NBS afforded highly reactive thial S-sulfides, which underwent dimerization to give the corresponding 1,2,4,5-tetrathianes. The products were also selectively converted into naturally-occurring cyclic polysulfides, 1,2,4-trithiolanes and 1,2,3,5,6-pentathiepanes, by treatment with Ph3P, KCN, or Na2S4.
-
Investigation on the Head-Space of Roasted Meat. III. Synthesis of 4, 6-dimethyl-2,3,5,7-tetrathiaoctane作者:Paul Dubs、Martin JohoDOI:10.1002/hlca.19780610807日期:1978.12.13A synthesis of 4, 6-dimethyl-2,3,5,7-tetrathiaoctane (1), from 3,5-dimethyl-1,2,4-trithiolane (6), is described. Compound 1, possessing a unique structure was recently found to be a constituent of roasted pork meat.4,6二甲基- 2,3,5,7-tetrathiaoctane(的合成1),由3,5-二甲基-1,2,4- trithiolane(6),进行说明。最近发现,具有独特结构的化合物1是烤猪肉的成分。
-
Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: S: SVol.4a/b, 1.3.11.4, page 298 - 300作者:DOI:——日期:——
-
Aroma compounds generated from thermal reaction of l-ascorbic acid with l-cysteine作者:Ai-Nong Yu、Ai-Dong ZhangDOI:10.1016/j.foodchem.2010.01.049日期:2010.8The reaction of L-ascorbic acid with L-cysteine in heated aqueous solution (141 +/- 1 degrees C) at five different pH values (5.00, 6.00, 7.00, 8.00, or 9.00) for 2 h, resulted in the formation of a complex mixture of aroma volatiles. The volatile compounds generated were analysed by SPME-GC-MS. The results gave 43 aroma compounds. The reaction between L.-ascorbic acid and L-cysteine led mainly to the formation of alicyclic sulphur compounds, thiophenes, thienothiophenes, thiophenones, thiazoles and pyrazines, most of which contain sulphur. Many of these volatiles had meaty flavour. The origin of many of the compounds was explained. The studies showed that thienothiophenes and thienones were formed mainly at acidic pH. In contrast, higher pH values could promote the production of thiophenes, thiazoles and pyrazines. (C) 2010 Elsevier Ltd. All rights reserved.
-
Nixon, L. N.; Wong, E.; Johnson, C. B., Journal of Agricultural and Food Chemistry, 1979, vol. 27, p. 355 - 359作者:Nixon, L. N.、Wong, E.、Johnson, C. B.、Birch, E. J.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
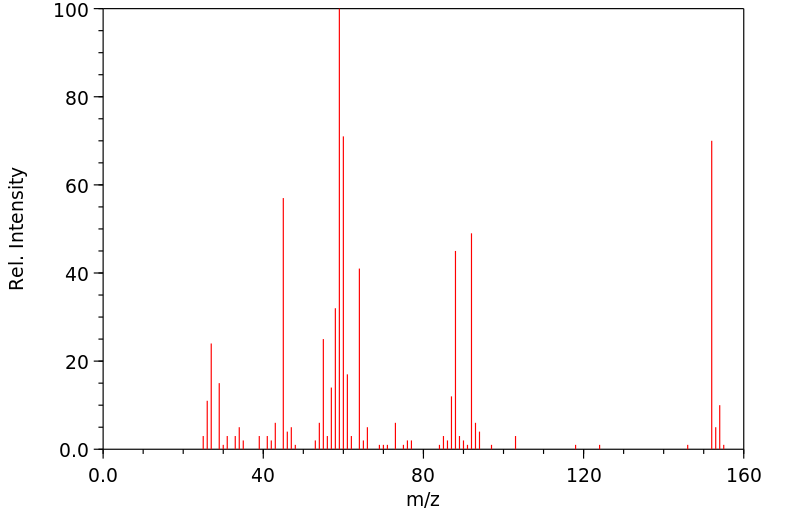
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
3,5-二甲基-1,2,4-三硫环戊烷
3,5-二异丁基-1,2,4-三硫环戊烷
3,5-二乙基-1,2,4-三硫杂环戊烷
3,5-二(1-甲基乙基)-1,2,4-三硫杂戊环
3,3,5,5-四甲基-1,2,4-三硫杂戊环
1,2,4-三硫杂戊环
1,1,3,3,7,7,9,9-Octamethyl-5,10,11-trithiadispiro<3.1.3.2>undecan-2,8-dithion
4-methyl-1,2,3-trithiolane
Tetrafluor-1,2,3-trithiolan
N,N,N',N'-tetra-tert-butyl-1,2,4-trithiolane-3,3,5,5-tetracarboxamide
3-Methyl-1,2,4-trithiolane
3,5-Ditert-butyl-1,2,4-trithiolane
spirocyclohexyl-1,2,4-trithiolane 4-S-oxide
spirocyclohexyl-1,2,4-trithiolane 1-S-oxide
cis-3,5-Dimethyl-1,2,4-trithiolan
trans-3,5-Diethyl-1,2,4-trithiolane
cis-3,5-Diethyl-1,2,4-trithiolan
2,2,8,8-tetrachloro-1,1,3,3,7,7,9,9-octamethyl-5,10,11-trithiadispiro[3.1.3.2]undecane
3,3,5,5-bis(pentamethylene)-1,2,4-trithiolane
trans-3,5-Dimethyl-1,2,4-trithiolan
(3S,5S)-3,5-Bis(2-methylpropyl)-1,2,4-trithiolane
(3R,5S)-3,5-Bis(2-methylpropyl)-1,2,4-trithiolane
3,5-Dibutyl-1,2,4-trithiolane
1,8-Dimethyl-2,6,9,12,13-pentathia-dispiro[4.1.4.2]tridecane
(3S,5R)-3-Ethyl-5-methyl-1,2,4-trithiolane
trans-3-Ethyl-5-methyl-1,2,4-trithiolane
1,2,3-trithiolane
3,5-Dimethyl-3,5-diaethyl-1,2,4-trithiolan
4-<(trimethylsilyl)methyl>-1,2,3-trithiacyclopentane
3,3,5,5-tetramethyl-1,2,4-trithiolane 4-oxide
3,3,5,5-tetramethyl-1,2,4-trithiolane 1-oxide
(1R,2R,6R,7R)-3,4,5-trithiatricyclo[5.2.1.02,6]dec-8-ene
1,2,4-Trithiolane, 3,5-dimethyl, #1
2-(1,2,3-trithiaspiro[4.4]nonan-9-yl)ethanethioic S-acid
(3S)-3-methyl-1,2,4-trithiolane
(3R)-3-propan-2-yl-1,2,4-trithiolane
(3S)-3-propan-2-yl-1,2,4-trithiolane
(3R,5R)-3,5-bis(2-methylpropyl)-1,2,4-trithiolane
1,2,4-Trithiolane, 5-ethenyl-3-ethyl
(1R,2S,6R,7S)-3,4,5-trithiatricyclo[5.2.1.02,6]dec-8-ene
Trithiolan-4-ylphosphonic acid
(3R)-3-methyl-1,2,4-trithiolane
(3R)-3-methyl-1,2,4-trithiolane;(3S)-3-methyl-1,2,4-trithiolane
1,2,4-Trithiolane, 3-ethyl-5-(1-methylethyl), #1
1,2,4-Trithiolane, 3-methyl-5-(1-methylethyl), #1
4,6a-Dihydro-3aH-cyclopenta[d][1,2,3]trithiole
dispiro[adamantane-2,3'-(1,2,4)-trithiolane-5',2'-adamantane]
1,1,3,3,7,7,9,9-octamethyl-5,10,11-trithiadispiro<3.1.3.2>undecane-2,8-dione
trans-2,2'-(1,2,4-Trithiolan-3,5-diyliden)bis(3,3-dimethylbutyronitril)
3,5-diisopropylidene-1,2,4-trithiolane