1,2,4-三硫杂戊环 | 289-16-7
中文名称
1,2,4-三硫杂戊环
中文别名
——
英文名称
1,2,4-trithiolane
英文别名
1,2,4-Trithiolan;1,3,5-Trithiolan
CAS
289-16-7
化学式
C2H4S3
mdl
——
分子量
124.252
InChiKey
QHGFEUAAQKJXDI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
LogP:2.689 (est)
-
保留指数:1065;1073;1106
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:5
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:75.9
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2,4,5,7,9-pentathiadecane 155994-67-5 C5H12S5 232.48
反应信息
-
作为反应物:描述:1,2,4-三硫杂戊环 生成 硫甲醛参考文献:名称:Bock, Hans; Hirabayashi, Takakuni; Mohmand, Shamsher, Chemische Berichte, 1982, vol. 115, # 2, p. 492 - 503摘要:DOI:
-
作为产物:描述:二氯甲烷 在 sodium disulfide 作用下, 反应 24.0h, 生成 1,2,4-三硫杂戊环参考文献:名称:1,2,4-三硫戊环的微波光谱、结构和电偶极矩摘要:Etude de 8 especes isotopiques du trithiolanne-1,2,4(类似硫磺de l'ozonide d'ethylene)。La structure estdetermine par ajustement des moindres carres des moment d'inertie。讨论DOI:10.1021/ja00316a002
文献信息
-
Sulfur Compounds, 199. Novel Titanocene Thiolato Complexes and Their Application in Preparing New Sulfur‐Containing Heterocycles作者:Ralf Steudel、Monika Kustos、Vera Münchow、Ursula WestphalDOI:10.1002/cber.19971300614日期:1997.6Treatment of Cp2Ti(CO)2 (3) with the di- and polysulfanes 1,2,4-(CH2)2S3, C4S6 (9), C,7H10S3 (11), 1,2,4,6-(CH2)3S4 (16), C6H10S6(19), and S6 affords the titanocene chelate complexes Cp2TiS3(CH2)2 (8), (Cp2Ti)2C4S6 (10), Cp2TiS3C7H10 (13), Cp2TiS2C7H10 (14), Cp2TiS4(CH2)3 (17), Cp,2TiS6C6H6H10 (20), and Cp2TiS8 (23). 14 is also obtained from Cp2TiCl2 (1) and the geminal dithiol of norbornene. The analogous用二,1,2,4-(CH 2)2 S 3,C 4 S 6(9),C,7 H 10 S 3(11)和二硫醚处理Cp 2 Ti(CO)2(3) ,1,2,4,6-(CH 2)3 S 4(16),C 6 H 10 S 6(19)和S 6可提供钛茂螯合物Cp 2 TiS 3(CH 2)2(8),(Cp 2 Ti)2 C 4 S 6(10),Cp 2 TiS 3 C 7 H 10(13),Cp 2 TiS 2 C 7 H 10(14),Cp 2 TiS 4(CH 2)3(17),Cp,2 TiS 6 C 6 H 6 H 10(20)和Cp 2TiS 8(23)。还从Cp 2 TiCl 2(1)和降冰片烯的双生二硫醇获得14。与二环戊二烯的二硫醇的类似反应产生Cp 2 TiS 2 C 10 H 12(15)。在配位体转移反应,8分发生反应在SCL 2,得到1,2,3,5- tetrathiane(25),10提供了9上反应有Cl
-
ThioformaldehydeS-sulfide (Thiosulfine)作者:Grzegorz Mlostoń、Jaroslaw Romański、Hans Peter Reisenauer、Günther MaierDOI:10.1002/1521-3773(20010119)40:2<393::aid-anie393>3.0.co;2-j日期:2001.1.19Matrix isolation spectroscopy allows the direct identification of ylide 1 and its cyclic isomer 2. They were obtained by pyrolysis of 1,2,4-trithiolane under high vacuum; the cyclic compound forms from 1 by thermal ring closure in a kinetically controlled reaction.
-
Cyclothiomethylation of aryl hydrazines with formaldehyde and hydrogen sulfide作者:V. R. Akhmetova、G. R. Nadyrgulova、T. V. Tyumkina、Z. A. Starikova、D. G. Golovanov、M. Yu. Antipin、R. V. Kunakova、U. M. DzhemilevDOI:10.1007/s11172-006-0493-7日期:2006.10Cyclothiomethylation of phenyl hydrazine with CH2O and H2S in a ratio of 1: 3: 2 in an acidic medium (HCl) afforded previously unknown 3-phenyl-1,3,4-thiadiazolidine (35% yield) and N-phenyl(perhydro-1,3,5-dithiazin-5-yl)amine (35% yield). The analogous reaction in an alkaline medium (BuONa) produced N-phenyl(perhydro-1,3-thiazetidin-3-yl)amine (22% yield). The reaction of 1,2-diphenyl hydrazine with CH2O and H2S in an alkaline medium gave 1,2,4,5-tetraphenylhexahydro-1,2,4,5-tetrazine and previously unknown 3,4-diphenyl-1,3,4-thiadiazolidine and 5,6-diphenyltetrahydro-1,3,5,6-dithiadiazepine in 39 and 22% yields, respectively. Cyclothiomethylation of benzyl hydrazine afforded previously unknown bis[(6-benzyl-4,2,6-thiadiazolidin-2-yl)methyl] sulfide (60% yield) and N-benzyl(perhydro-1,3,5-dithiazin-5-yl)amine (19% yield). The reaction of tosyl hydrazine produced 3-[(p-tolyl)sulfonyl]-1,3,4-thiadiazolidine, N-(perhydro-1,3,5-dithiazin-5-yl)-p-tolylsulfonamide, and 3,7-bis(p-tolylsulfonylamino)-1,5-dithia-3,7-diazacyclooctane in 21, 38, and 41% yields, respectively.在酸性介质(HCl)中,以1:3:2的比例将氨基苯肼与CH2O和H2S进行环硫甲基化反应,得到之前未报道的3-苯基-1,3,4-噻二唑烷(产率35%)和N-苯基(全氢-1,3,5-二硫唑-5-基)胺(产率35%)。在碱性介质(BuONa)中进行类似反应则产生了N-苯基(全氢-1,3-噻唑烷-3-基)胺(产率22%)。1,2-二苯基肼与 和 在碱性介质中反应得到了1,2,4,5-四苯基六氢-1,2,4,5-四氮杂烯以及之前未知的3,4-二苯基-1,3,4-噻二唑烷和5,6-二苯基四氢-1,3,5,6-二噻二氮烯,产率分别为39%和22%。苄基肼的环硫甲基化反应产生了之前未知的双[(6-苄基-4,2,6-噻二唑烷-2-基)甲基]硫(产率60%)和N-苄基(全氢-1,3,5-二硫唑-5-基)胺(产率19%)。对磺酰肼的反应产生了3-[(对甲苯基)磺酰]-1,3,4-噻二唑烷、N-(全氢-1,3,5-二硫唑-5-基)-对甲苯基磺酰胺以及3,7-二(对甲苯基磺酰胺)-1,5-二硫-3,7-二氮杂环辛烷,产率分别为21%、38%和41%。
-
Multicomponent heterocyclization of carboxamides with H2S and CH2O作者:V. R. Akhmetova、R. R. Khairullina、G. R. Nadyrgulova、R. V. Kunakova、U. M. DzhemilevDOI:10.1134/s1070428008020036日期:2008.2Multicomponent heterocyclization of aliphatic amides with H2S and CH2O (1:3:2) in water-organic solvent mixture in the presence of BuONa led to the formation of 1,3,5-dithiazinane in high yield (30–95%) and with high selectivity (100%). Under these conditions benzamide gave 3,5-dibenzoyl-1,3,5-thiadiazinane in 74% yield, whereas due to ortho-effect the acetylsalicylamide with H2S and CH2O in a system BuOH-H2O without BuONa formed N-acetylsalicyloyl-1,3,5-dithiazinane (80%). Heterocyclization of α-aminosuccinic acid monoamide depending on the H2S and CH2O concentration occurred either at one or both NH2 yielding respectively mono-or bisdithiazinanes.
-
Reactions of 1,2,4‐Trithiolanes with Nonacarbonyldiiron: Sulfurdithiolatodiiron and ‐tetrairon Complexes as Mimics for the Active Site of [Fe‐only] Hydrogenase作者:Jochen Windhager、Helmar Görls、Holm Petzold、Grzegorz Mloston、Gerald Linti、Wolfgang WeigandDOI:10.1002/ejic.200700465日期:2007.104)-trithiolane-5′,2′-tricyclo[3.3.1.1]decane} (1e) with nonacarbonyldiiron (2) have been investigated. The sulfurdithiolatodiiron complexes 3a–e, which can be considered as novel model complexes of the active site of the [Fe-only] hydrogenase, were isolated as the main products of these reactions. X-ray structure analyses were performed on compounds 3b–e. The carbon dithiolato (SCR2S)-bridged diiron side-products1,2,4-trithiolane (1a), 3,3,5,5-tetramethyl-1,2,4-trithiolane (1b), 3,3,5,5-tetraethyl-1,2,4 的反应-trithiolane (1c), 3,3,5,5-bis(pentaethylene)-1,2,4-trithiolane (1d) 和 di-spirotricyclo[3.3.1.1]decane-2,3″-(1, 2,4)-trithiolane-5',2'-tricyclo[3.3.1.1]decane} (1e) 与 nonacarbonyldiiron (2) 已被研究。硫二硫基二铁配合物 3a-e 可以被认为是 [Fe-only] 氢化酶活性位点的新型模型配合物,作为这些反应的主要产物被分离出来。对化合物 3b-e 进行 X 射线结构分析。还获得了二硫代碳(SCR2S)桥接的二铁副产物
表征谱图
-
氢谱1HNMR
-
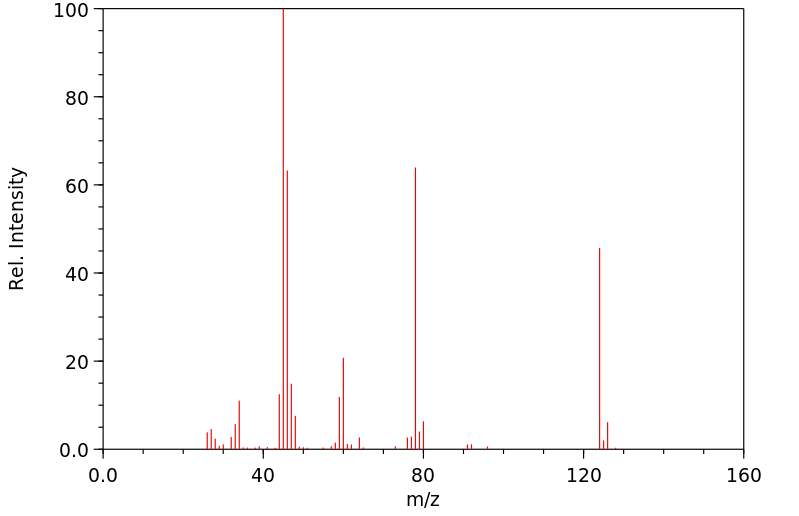
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
3,5-二甲基-1,2,4-三硫环戊烷
3,5-二异丁基-1,2,4-三硫环戊烷
3,5-二乙基-1,2,4-三硫杂环戊烷
3,5-二(1-甲基乙基)-1,2,4-三硫杂戊环
3,3,5,5-四甲基-1,2,4-三硫杂戊环
1,2,4-三硫杂戊环
1,1,3,3,7,7,9,9-Octamethyl-5,10,11-trithiadispiro<3.1.3.2>undecan-2,8-dithion
4-methyl-1,2,3-trithiolane
Tetrafluor-1,2,3-trithiolan
N,N,N',N'-tetra-tert-butyl-1,2,4-trithiolane-3,3,5,5-tetracarboxamide
3-Methyl-1,2,4-trithiolane
3,5-Ditert-butyl-1,2,4-trithiolane
spirocyclohexyl-1,2,4-trithiolane 4-S-oxide
spirocyclohexyl-1,2,4-trithiolane 1-S-oxide
cis-3,5-Dimethyl-1,2,4-trithiolan
trans-3,5-Diethyl-1,2,4-trithiolane
cis-3,5-Diethyl-1,2,4-trithiolan
2,2,8,8-tetrachloro-1,1,3,3,7,7,9,9-octamethyl-5,10,11-trithiadispiro[3.1.3.2]undecane
3,3,5,5-bis(pentamethylene)-1,2,4-trithiolane
trans-3,5-Dimethyl-1,2,4-trithiolan
(3S,5S)-3,5-Bis(2-methylpropyl)-1,2,4-trithiolane
(3R,5S)-3,5-Bis(2-methylpropyl)-1,2,4-trithiolane
3,5-Dibutyl-1,2,4-trithiolane
1,8-Dimethyl-2,6,9,12,13-pentathia-dispiro[4.1.4.2]tridecane
(3S,5R)-3-Ethyl-5-methyl-1,2,4-trithiolane
trans-3-Ethyl-5-methyl-1,2,4-trithiolane
1,2,3-trithiolane
3,5-Dimethyl-3,5-diaethyl-1,2,4-trithiolan
4-<(trimethylsilyl)methyl>-1,2,3-trithiacyclopentane
3,3,5,5-tetramethyl-1,2,4-trithiolane 4-oxide
3,3,5,5-tetramethyl-1,2,4-trithiolane 1-oxide
(1R,2R,6R,7R)-3,4,5-trithiatricyclo[5.2.1.02,6]dec-8-ene
1,2,4-Trithiolane, 3,5-dimethyl, #1
2-(1,2,3-trithiaspiro[4.4]nonan-9-yl)ethanethioic S-acid
(3S)-3-methyl-1,2,4-trithiolane
(3R)-3-propan-2-yl-1,2,4-trithiolane
(3S)-3-propan-2-yl-1,2,4-trithiolane
(3R,5R)-3,5-bis(2-methylpropyl)-1,2,4-trithiolane
1,2,4-Trithiolane, 5-ethenyl-3-ethyl
(1R,2S,6R,7S)-3,4,5-trithiatricyclo[5.2.1.02,6]dec-8-ene
Trithiolan-4-ylphosphonic acid
(3R)-3-methyl-1,2,4-trithiolane
(3R)-3-methyl-1,2,4-trithiolane;(3S)-3-methyl-1,2,4-trithiolane
1,2,4-Trithiolane, 3-ethyl-5-(1-methylethyl), #1
1,2,4-Trithiolane, 3-methyl-5-(1-methylethyl), #1
4,6a-Dihydro-3aH-cyclopenta[d][1,2,3]trithiole
dispiro[adamantane-2,3'-(1,2,4)-trithiolane-5',2'-adamantane]
1,1,3,3,7,7,9,9-octamethyl-5,10,11-trithiadispiro<3.1.3.2>undecane-2,8-dione
trans-2,2'-(1,2,4-Trithiolan-3,5-diyliden)bis(3,3-dimethylbutyronitril)
3,5-diisopropylidene-1,2,4-trithiolane