N,N-Dimethyl-N'-p-fluorophenyl-thioharnstoff | 710-21-4
中文名称
——
中文别名
——
英文名称
N,N-Dimethyl-N'-p-fluorophenyl-thioharnstoff
英文别名
3-(p-Fluorophenyl)-1,1-dimethylthiourea;3-(4-fluorophenyl)-1,1-dimethylthiourea
CAS
710-21-4
化学式
C9H11FN2S
mdl
MFCD02827999
分子量
198.264
InChiKey
KLZBBZDRSCOSOH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:13
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:47.4
-
氢给体数:1
-
氢受体数:2
反应信息
-
作为反应物:描述:N,N-Dimethyl-N'-p-fluorophenyl-thioharnstoff 在 吡啶 、 碘苯二乙酸 、 nickel dibromide 作用下, 以 1,4-二氧六环 为溶剂, 反应 0.17h, 以86%的产率得到6-fluoro-N,N-dimethylbenzo[d]thiazol-2-amine参考文献:名称:通过镍催化的分子内氧化CH官能团轻度合成2-取代的苯并噻唑。摘要:已经报道了从芳基硫脲开始制备2-氨基苯并噻唑的高效合成方法。通过使用镍催化剂,芳基硫脲进行分子内氧化CH键官能化,以良好至优异的产率得到所需的2-氨基苯并噻唑。该方案具有廉价的催化剂,催化剂用量低,反应条件温和,反应时间短,收率良好至优异的特点,并且可以轻松按比例放大至克级,而收率几乎没有下降。DOI:10.1021/acs.joc.9b02543
-
作为产物:描述:参考文献:名称:芳烃插入硫脲:一种新的Am合成。摘要:由三甲基甲硅烷基苯基三氟甲磺酸酯前体生成的芳烃已通过与π插入C═S键的形式与硫脲反应。该反应与尿素的反应形成对比,后者是通过将苯乙炔σ插入C–N键进行的,代表了一条新的,操作简单的途径来官能化am。DOI:10.1021/ol202049v
文献信息
-
Facile synthesis of S -arylisothioureas via the S -arylation of arylthioureas with aryl iodides in water作者:Xing Liu、Hui Zhu、Shi-Bo Zhang、Yu Cheng、Han-Ying Peng、Zhi-Bing DongDOI:10.1016/j.tetlet.2018.07.012日期:2018.8An environmentally benign, simple and highly efficient protocol for the synthesis of S-arylisothiourea derivatives has been achieved in good to excellent yields by reacting a series of aryl iodides with arylthioureas, using inexpensive CuSO4·5H2O as catalyst in water without PTC (phase transfer catalyst). The protocol features easy performance, good to excellent yields, good tolerance towards a variety
-
Copper-Catalyzed S-Arylation of Arylthioureas by Using Diaryliodonium Salts作者:Yue-Sheng Li、Zhi-Bing Dong、Hui Zhu、Xing Liu、Yu Cheng、Han-Ying PengDOI:10.1055/s-0036-1591569日期:2018.6and pharmaceutical compounds. A convenient and efficient copper-catalyzed S-arylation of arylthioureas using diaryliodonium salts is reported. The desired arylisothioureas were synthesized in good yields in the presence of CuCl as catalyst and K2CO3 as base, and a wide variety of functional groups on the arylthioureas and diaryliodonium salts were tolerated. The protocol affords an alternative synthesis
-
An Efficient Chan-Lam <i>S</i> -Arylation of Arylthioureas with Arylboronic Acids作者:Xing Liu、Shi-Bo Zhang、Hui Zhu、Yu Cheng、Han-Ying Peng、Zhi-Bing DongDOI:10.1002/ejoc.201800892日期:2018.8.31A convenient and efficient protocol has been developed for the preparation of aryl-isothioureas based on the Chan-Lam C-S coupling reaction. The desired aryl-isothioureas were synthesized in good yields (up to 95%) in the presence of Cu(OAc)(2)H2O as catalyst and bipyridine as ligand. A variety of functional groups on the aryl-thiourea and arylboronic acid reagents are tolerated. The method features
-
Copper-Catalyzed and Air-Mediated Mild Cross-Dehydrogenative Coupling of Aryl Thioureas and Dialkyl<i>H</i>-Phosphonates: The Synthesis of Thiophosphonates作者:Cai-Zhu Chang、Xing Liu、Hui Zhu、Li Wu、Zhi-Bing DongDOI:10.1021/acs.joc.8b02011日期:2018.11.2A copper-catalyzed and air-mediated mild cross-dehydrogenative coupling of aryl thioureas and dialkyl H-phosphonates to produce thiophosphonates has been reported. Without addition of base or acid, air as an oxidant, the phosphorylation occurred smoothly to give the target products in moderate to excellent yields at room temperature. The protocol allows the direct formation of a sulfur–phosphorus bond
-
Cs<sub>2</sub>CO<sub>3</sub>-Promoted Hydrothiolation of Alkynes with Aryl Thioureas: Stereoselective Synthesis of (<i>Z</i>)-Vinyl Sulfides作者:Dan Wang、Han-Ying Peng、Meng-Meng Yang、Er-jun Hao、Yue-Sheng Li、Zhi-Bing DongDOI:10.1021/acs.joc.1c00309日期:2021.6.18thioureas for stereoselective synthesis of (Z)-vinyl sulfides has been reported. Vinyl thioethers were obtained without a metal catalyst in good yields via anti-Markovnikov and cis addition. The protocol features a broad substrate scope of the starting materials, high atom economy, good yields, and exclusive stereoselectivity, showing potential synthetic value for the synthesis of a diversity of (Z)-vinyl
表征谱图
-
氢谱1HNMR
-
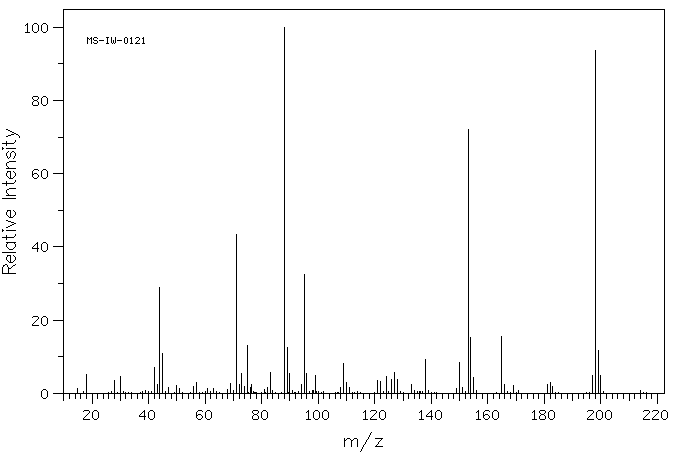
质谱MS
-
碳谱13CNMR
-
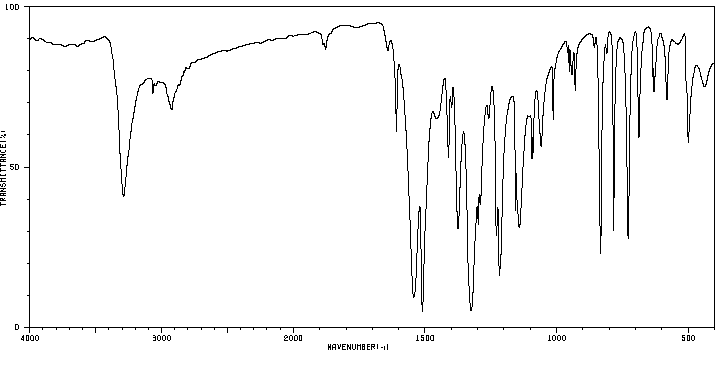
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫