3-氯-2-甲基噻吩 | 17249-83-1
中文名称
3-氯-2-甲基噻吩
中文别名
——
英文名称
3-chloro-2-methylthiophene
英文别名
3-chloro-2-methyl thiophene;2-methyl-3-chlorothiophene
CAS
17249-83-1
化学式
C5H5ClS
mdl
——
分子量
132.614
InChiKey
NUFSHDCNQRBKRK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:28.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险类别:3
-
危险性防范说明:P501,P240,P210,P233,P243,P241,P242,P264,P280,P370+P378,P337+P313,P305+P351+P338,P362+P364,P303+P361+P353,P332+P313,P403+P235
-
危险品运输编号:1993
-
危险性描述:H315,H319,H225
-
包装等级:III
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲基噻吩 2-Methylthiophene 554-14-3 C5H6S 98.1686
反应信息
-
作为反应物:描述:二氧化碳 、 3-氯-2-甲基噻吩 、 三甲基硅烷化重氮甲烷 在 1,3-二甲基-2-咪唑啉酮 、 lithium 3-ethylpentan-3-olate 、 cesium fluoride 作用下, 以 甲醇 、 正己烷 为溶剂, 150.0 ℃ 、101.33 kPa 条件下, 以62 %的产率得到methyl 4-chloro-5-(2-methoxy-2-oxoethyl)thiophene-2-carboxylate参考文献:名称:1,5-Double-Carboxylation of 2-Alkylheteroarenes Mediated by a Combined Brønsted Base System摘要:摘要 本文报告了一种组合式勃朗斯特碱(t-BuOLi/CsF 或 LiOCEt3/CsF)体系在苄基和 δ 位介导非融合 2-烷基噻吩的 1,5-双羧化反应。在既定的反应条件下,可以容许多种官能团(OMe、F、Cl、CF3、OCF3、硫化物、CN、酰胺、酮或砜)。DOI:10.1055/a-1990-5360
-
作为产物:描述:参考文献:名称:Biphasic electrophilic halogenation of activated aromatics and heteroaromatics with N-halosuccinimides catalyzed by perchloric acid摘要:Catalytic amounts of 70% perchloric acid (0.1-10, mostly 0.1-1, mol %, based on substrate) initiate the regioselective halogenation of activated aromatics [mesitylene, 1,3-dimethoxybenzene, 2,3-dimethylanisole, o-xylene] and heteroaromatics [thiophene, its 2-methyl, 2-halo (chloro, bromo, iodo), and 3-bromo derivatives] with N-halosuccinimide (NXS, X = Cl or Br) in two-phase solid-liquid systems (NXS/hexane or NXS/CCl4) at room temperature to give ring-halogenated products in high yields. For example, thiophene is transformed to 2-halo or 2,5-dihalo derivatives (yield 82-98%) using 1 or 2 equiv of NXS, respectively. Unsymmetrical 2,5-dihalothiophenes are obtained in 70-82% yield by reacting 2-halothiophenes with an appropriate NXS. The reaction of 3-bromothiophene with NBS affords 2,3-dibromothiophene in 93-99% yield. 1,3-Dimethoxybenzene and 2,3-dimethylanisole are halogenated regiospecifically at the 4 position to give the corresponding products in 81-94% yield.DOI:10.1021/jo00063a028
文献信息
-
[EN] HETEROCYCLIC COMPOUNDS FOR THE TREATMENT OF STRESS-RELATED CONDITIONS<br/>[FR] COMPOSÉS HÉTÉROCYCLIQUES POUR LE TRAITEMENT D'ÉTATS LIÉS AU STRESS申请人:OTSUKA PHARMA CO LTD公开号:WO2010137738A1公开(公告)日:2010-12-02The present invention provides a novel heterocyclic compound. A heterocyclic compound represented by general formula (1) wherein, R1 and R2, each independently represent hydrogen; a phenyl lower alkyl group that may have a substituent(s) selected from the group consisting of a lower alkyl group and the like on a benzene ring and/or a lower alkyl group; or a cyclo C3-C8 alkyl lower alkyl group; or the like; R3 represents a lower alkynyl group or the like; R4 represents a phenyl group that may have a substituent(s) selected from the group consisting of a 1,3,4-oxadiazolyl group that may have e.g., halogen or a heterocyclic group selected from pyridyl group and the like; the heterocyclic group may have at least one substituent(s) selected from a lower alkoxy group and the like or a salt thereof.
-
POLYSUBSTITUTED DERIVATIVES OF 2-HETEROARYL-6-PHENYLIMIDAZO[1,2-a]PYRIDINES, AND PREPARATION AND THERAPEUTIC USE THEREOF申请人:DE PERETTI Danielle公开号:US20110065727A1公开(公告)日:2011-03-17Compounds of formula (I): wherein R, R 1 , R 2 , R 3 , R 4 and X are as defined in the disclosure, or an acid addition salt thereof, and the therapeutic use and process of synthesis thereof.式(I)的化合物: 其中R、R1、R2、R3、R4和X如披露中所定义,或其酸盐,以及其治疗用途和合成过程。
-
[EN] COT MODULATORS AND METHODS OF USE THEREOF<br/>[FR] MODULATEURS DE COT ET LEURS PROCÉDÉS D'UTILISATION申请人:GILEAD SCIENCES INC公开号:WO2020252151A1公开(公告)日:2020-12-17The present disclosure relates generally to modulators of Cot (cancer Osaka thyroid) of generic Formula (I) and methods of use and manufacture thereof.本公开涉及一般性地与Cot(癌症大阪甲状腺)的通用化学式(I)的调节剂,以及其使用和制造方法。
-
[EN] COMPOUNDS AND USES THEREOF<br/>[FR] COMPOSÉS ET UTILISATIONS DE CES DERNIERS申请人:YUMANITY THERAPEUTICS公开号:WO2018081167A1公开(公告)日:2018-05-03The present invention features compounds useful in the treatment of neurological disorders. The compounds of the invention, alone or in combination with other pharmaceutically active agents, can be used for treating or preventing neurological disorders.本发明涉及对神经系统疾病治疗中有用的化合物。本发明的化合物,单独或与其他药用活性剂结合,可用于治疗或预防神经系统疾病。
-
HETEROCYCLIC CETP INHIBITORS申请人:Salvati E. Mark公开号:US20070161685A1公开(公告)日:2007-07-12Compounds of formula Ia and Ib wherein A, B, C and R 1 are described herein.式Ia和Ib的化合物 其中A、B、C和R1 如本文所述。
表征谱图
-
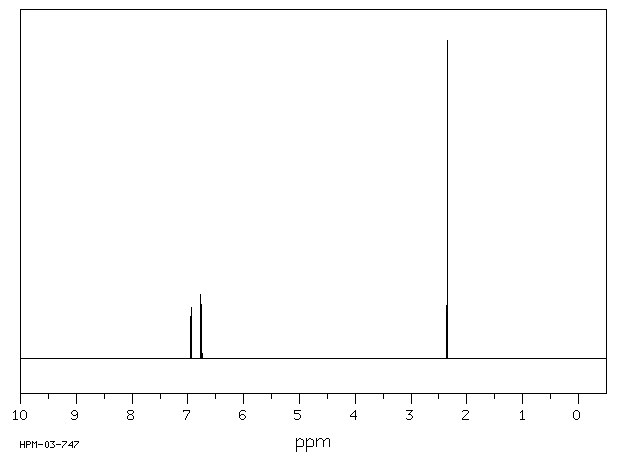
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
试剂2,5-Dibromo-3,4-dihexylthiophene
苯-1,2,4-三羧酸-丙烷-1,2,3-三醇(1:1)
碘吡咯
癸氯-二茂铁
甲酮,(4,5-二溴-1H-吡咯-2-基)苯基-
甲基3-氟-1H-1,2,4-三唑-5-羧酸酯
溴代二茂铁
溴-(3-溴-2-噻嗯基)镁
派瑞林D
派瑞林 F 二聚体
氯代二茂铁
曲洛酯
异噻唑,3-氯-5-甲基-
地茂酮
四碘硒吩
四碘噻吩
四碘呋喃
四溴噻吩
四溴吡咯
四溴-N-甲基吡咯
四氯噻吩
四氟噻吩
噻菌腈
噻美尼定.
噻吩,3-溴-4-(1-辛炔基)-
噻吩,3-溴-2-[2-(甲硫基)乙烯基]-,(Z)-
噻吩,3-溴-2-[2-(甲硫基)乙烯基]-,(E)-
噻吩,3-溴-2-[2-(甲硫基)乙烯基]-,(E)-
噻吩,2,5-二氯-3,4-二(氯甲基)-
喷贝特
咪唑烷,2-(4-溴-5-甲基-2-呋喃基)-1,3-二甲基-
叔丁基2-溴-4,6-二氢-5H-吡咯并[3,4-D]噻唑-5-羧酸酯
叔-丁基3-溴-6,7-二氢-1H-吡唑并[4,3-C]吡啶-5(4H)-甲酸基酯
叔-丁基2-溴-5,6-二氢咪唑并[1,2-A]吡嗪-7(8H)-甲酸基酯
叔-丁基(4-溴-5-氰基-1-甲基-1H-吡唑-3-基)氨基甲酯
双环[4.2.0]辛-1,3,5-三烯-7-甲腈,2-氟-
八氟联苯烯
八氟二苯并硒吩
全氟苯并环丁烯二酮
二苯基氯化碘盐
二联苯碘硫酸盐
二氯对二甲苯二聚体
二氯[2-甲基-3(2H)-异噻唑酮-O]的钙合物
二氯-1,2-二硫环戊烯酮
二-(3-溴-1,2,4-噻二唑-5-基)-二硫醚
二(2-噻吩基)碘鎓
乙酸,[[[1-(3-溴-5-异[口噁]唑基)亚乙基]氨基]氧代]-,甲基酯,(E)-
[四丁基铵][Δ-三(四氯-1,2-苯二醇酸根)磷酸盐(V)]
[3-(4-氯-3,5-二甲基-1H-吡唑-1-基)丙基]胺
[3-(4-氯-1H-吡唑-1-基)-2-甲基丙基]胺