3-甲基-2-(甲硫基)苯并噻唑鎓对甲苯磺酸盐 | 55514-14-2
中文名称
3-甲基-2-(甲硫基)苯并噻唑鎓对甲苯磺酸盐
中文别名
——
英文名称
3-methyl-2-(methylthio)benzothiazolium tosylate
英文别名
3-methyl-2-(methylthio)benzothiazolium p-toluenesulfonate;3-methyl-2-(methylthio)benzothiazol-3-ium p-toluenesulfonate;3-Methyl-2-(methylthio)benzo[d]thiazol-3-ium 4-methylbenzenesulfonate;4-methylbenzenesulfonate;3-methyl-2-methylsulfanyl-1,3-benzothiazol-3-ium
CAS
55514-14-2
化学式
C7H7O3S*C9H10NS2
mdl
——
分子量
367.514
InChiKey
SGABZOJLSUJGFH-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.35
-
重原子数:23
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.19
-
拓扑面积:123
-
氢给体数:0
-
氢受体数:5
安全信息
-
储存条件:2-8°C,惰性气体
SDS
反应信息
-
作为反应物:描述:3-甲基-2-(甲硫基)苯并噻唑鎓对甲苯磺酸盐 在 三乙胺 作用下, 以 N,N-二甲基甲酰胺 、 乙腈 为溶剂, 反应 4.0h, 生成 (2Z,5E)-3-ethyl-5-(3-methyl-1,3-benzothiazol-2-ylidene)-2-[(1-methylquinolin-1-ium-2-yl)methylidene]-1,3-thiazolidin-4-one;4-methylbenzenesulfonate参考文献:名称:Rhodacyanine Dyes as Antimalarials. 1. Preliminary Evaluation of Their Activity and Toxicity摘要:The rhodacyanine dye MKT-077 (1), a potent antitumor agent, was found to possess strong in vitro activity against Plasmodium falciparum and a low cytotoxicity. Several new rhodacyanine dyes related to 1, containing a variety of linked heterocyclic moieties, were synthesized, and their antimalarial potencies were evaluated. The synthetic rhodacyanines were found to have EC50 values against P. falciparum in vitro in the range of 4-300 nM. Several compounds in this series have remarkable selective toxicity profiles (> 100).DOI:10.1021/jm0155704
-
作为产物:描述:参考文献:名称:375.热量对含有烷硫基的杂环碱基的作用。第一部分.2-烷基硫代苯并噻唑和2-烷基硫代-苯并恶唑摘要:DOI:10.1039/jr9510001723
文献信息
-
2-[3H-THIAZOL-2-YLIDINEMETHYL]PYRIDINES AND RELATED COMPOUNDS AND THEIR USE申请人:Brown Robert公开号:US20090247579A1公开(公告)日:2009-10-01The present invention pertains to certain 2-[3H-thiazol-2-ylidinemethyl]pyridine compounds and analogs thereof, which, inter alia, inhibit cell proliferation, treat cancer, etc., and more specifically to compounds of the following formula, wherein R A1 , R A2 , R A3 , R A4 , R B1 , R B2 , R NA , R NB , and X − are as defined herein: The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit cell proliferation, and in the treatment of proliferative conditions such as cancer, etc.
-
Synthesis of three classes of rhodacyanine dyes and evaluation of their in vitro and in vivo antimalarial activity作者:Khanitha Pudhom、Kazuki Kasai、Hiroki Terauchi、Hiroshi Inoue、Marcel Kaiser、Reto Brun、Masataka Ihara、Kiyosei TakasuDOI:10.1016/j.bmc.2006.08.035日期:2006.120]-rhodacyanines, were synthesized and their in vitro antimalarial activities against Plasmodium falciparum K1 (chloroquine-resistant strain) as well as their in vivo activities against P. berghei in mice were determined. The novel [0,0,0]-rhodacynines, 3e and 3h, possessing a benzothiazole moiety, were shown to have highly promising antimalarial activities in vivo. Moreover, the [0,0,0]-rhodacyanines were found
-
Nucleic acid probes and methods to detect and/or quantify nucleic acid analytes申请人:PROLIGO, LLC公开号:US20030143591A1公开(公告)日:2003-07-31The invention comprises novel methods and strategies to detect and/or quantify nucleic acid analytes. The methods involve nucleic acid probes with covalently conjugated dyes, which are attached either at adjacent nucleotides or at the same nucleotide of the probe and novel linker molecules to attach the dyes to the probes. The nucleic acid probes generate a fluorescent signal upon hybridization to complementary nucleic acids based on the interaction of one of the attached dyes, which is either an intercalator or a DNA groove binder, with the formed double stranded DNA. The methods can be applied to a variety of applications including homogeneous assays, real-time PCR monitoring, transcription assays, expression analysis on nucleic acid microarrays and other microarray applications such as genotyping (SNP analysis). The methods further include pH-sensitive nucleic acid probes that provide switchable fluorescence signals that are triggered by a change in the pH of the medium.
-
Novel 3-Methyl-2-alkylthio Benzothiazolyl-Based Ionic Liquids: Synthesis, Characterization, and Antibiotic Activity作者:Teng Zhang、Hao He、Jun Du、Zhi He、Shun YaoDOI:10.3390/molecules23082011日期:——first time. After structural identification, their melting point, solubility, and thermostability together with antibiotic activity were determined successively. As a result, 3-methyl-2-alkylthio benzothiazolyl p-toluene sulfonate was found to have the highest antibacterial activity among the three series of ILs. Meanwhile, it has a good solubility in water as well. On the basis of comprehensive comparison
-
Heterocyclic modulators of nuclear receptors申请人:——公开号:US20030212111A1公开(公告)日:2003-11-13Compounds, compositions and methods for modulating the activity of nuclear receptors are provided. In particular, heterocyclic compounds are provided for modulating the activity of farnesoid X receptor (FXR), liver X receptor (LXR) and/or orphan nuclear receptors. In certain embodiments, the compounds are thiazolidinone derivatives.
表征谱图
-
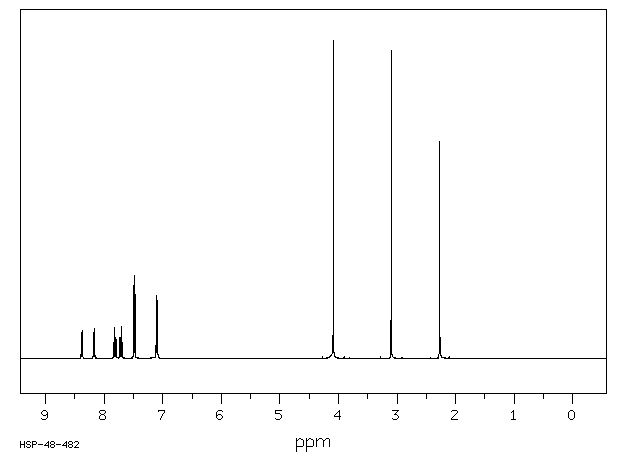
氢谱1HNMR
-
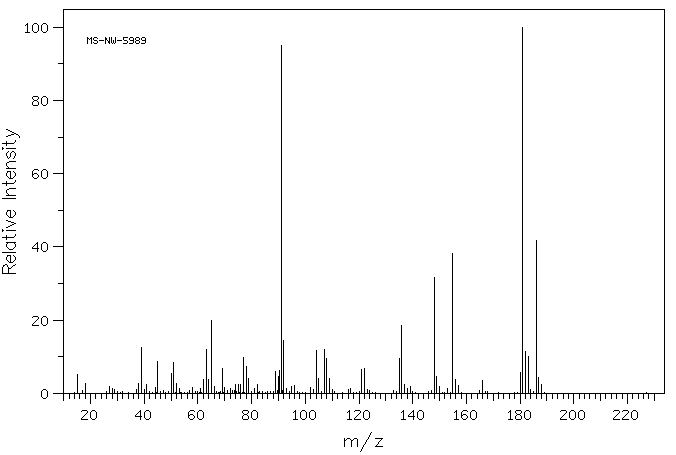
质谱MS
-
碳谱13CNMR
-
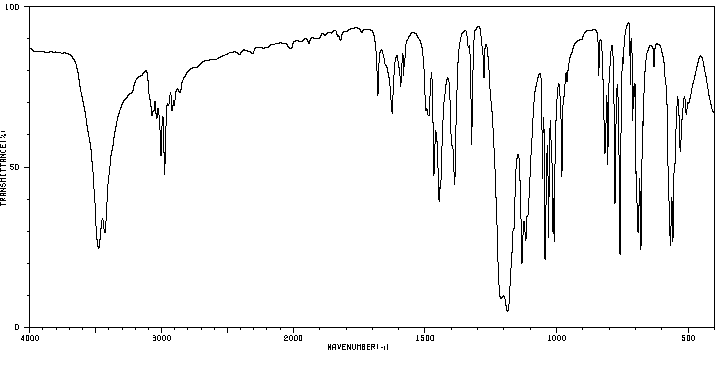
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(1Z)-1-(3-乙基-5-羟基-2(3H)-苯并噻唑基)-2-丙酮
齐拉西酮砜
齐帕西酮-d8
阳离子蓝NBLH
阳离子荧光黄4GL
锂2-(4-氨基苯基)-5-甲基-1,3-苯并噻唑-7-磺酸酯
铜酸盐(4-),[2-[2-[[2-[3-[[4-氯-6-[乙基[4-[[2-(硫代氧代)乙基]磺酰]苯基]氨基]-1,3,5-三嗪-2-基]氨基]-2-(羟基-kO)-5-硫代苯基]二氮烯基-kN2]苯基甲基]二氮烯基-kN1]-4-硫代苯酸根(6-)-kO]-,(1:4)氢,(SP-4-3)-
铜羟基氟化物
钾2-(4-氨基苯基)-5-甲基-1,3-苯并噻唑-7-磺酸酯
钠3-(2-{(Z)-[3-(3-磺酸丙基)-1,3-苯并噻唑-2(3H)-亚基]甲基}[1]苯并噻吩并[2,3-d][1,3]噻唑-3-鎓-3-基)-1-丙烷磺酸酯
邻氯苯骈噻唑酮
西贝奈迪
螺[3H-1,3-苯并噻唑-2,1'-环戊烷]
螺[3H-1,3-苯并噻唑-2,1'-环己烷]
葡萄属英A
草酸;N-[1-[4-(2-苯基乙基)哌嗪-1-基]丙-2-基]-2-丙-2-基氧基-1,3-苯并噻唑-6-胺
苯酰胺,N-2-苯并噻唑基-4-(苯基甲氧基)-
苯酚,3-[[2-(三苯代甲基)-2H-四唑-5-基]甲基]-
苯胺,N-(3-苯基-2(3H)-苯并噻唑亚基)-
苯碳杂氧杂脒,N-1,2-苯并异噻唑-3-基-
苯甲酸,4-(6-辛基-2-苯并噻唑基)-
苯甲基2-甲基哌啶-1,2-二羧酸酯
苯并噻唑正离子,2-[3-(1,3-二氢-1,3,3-三甲基-2H-吲哚-2-亚基)-1-丙烯-1-基]-3-乙基-,碘化(1:1)
苯并噻唑正离子,2-[2-[4-(二甲氨基)苯基]乙烯基]-3-乙基-6-甲基-,碘化
苯并噻唑正离子,2-[(2-乙氧基-2-羰基乙基)硫代]-3-甲基-,溴化
苯并噻唑啉
苯并噻唑三氯金(III)
苯并噻唑-d4
苯并噻唑-7-乙酸
苯并噻唑-6-腈
苯并噻唑-5-羧酸
苯并噻唑-5-硼酸频哪醇酯
苯并噻唑-4-醛
苯并噻唑-4-乙酸
苯并噻唑-2-磺酸钠
苯并噻唑-2-磺酸
苯并噻唑-2-磺酰氟
苯并噻唑-2-甲醛
苯并噻唑-2-甲酸
苯并噻唑-2-甲基甲胺
苯并噻唑-2-基磺酰氯
苯并噻唑-2-基甲基-乙基-胺
苯并噻唑-2-基叠氮化物
苯并噻唑-2-基-邻甲苯-胺
苯并噻唑-2-基-己基-胺
苯并噻唑-2-基-(4-氯-苯基)-胺
苯并噻唑-2-基-(4-氟-苯基)-胺
苯并噻唑-2-基-(4-乙氧基-苯基)-胺
苯并噻唑-2-基-(2-甲氧基-苯基)-胺
苯并噻唑-2-基-(2,6-二甲基-苯基)-胺