3-甲氧基-25-二甲基吡嗪 | 19846-22-1
中文名称
3-甲氧基-25-二甲基吡嗪
中文别名
——
英文名称
3-methoxyl-2,5-dimethylpyrazine
英文别名
2-methoxy-3,6-dimethylpyrazine;3,6-dimethyl-2-methoxypyrazine;3-methoxy-2,5-dimethylpyrazine;3-methoxy-2,5-dimethyl-pyrazine;2,5-dimethyl-3-methoxypyrazine;3-Methoxy-2,5-dimethyl-pyrazin
CAS
19846-22-1
化学式
C7H10N2O
mdl
MFCD28162737
分子量
138.169
InChiKey
QYRGVELVPYDICQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:179.3±35.0 °C(Predicted)
-
密度:1.043±0.06 g/cm3(Predicted)
-
LogP:1.670 (est)
-
保留指数:1054;1057;1065
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.428
-
拓扑面积:35
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2933990090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
SDS
反应信息
-
作为反应物:描述:参考文献:名称:The Synthesis of Pyrrolo[1,2-a]pyrazin-1(2H)-ones and Pyrrolo[1,2-b]pyridazin-6(5H)-ones摘要:The pyrrolo[1,2-a]pyrazin-1(2H)-ones (10), (11), (12) and (13) and the pyrrolo[1,2-b[pyridazin-6(5H)-one (18) were prepared either a) directly by Chichibabin quaternisation-cyclisation of the corresponding methoxy-methylpyrazine or pyridazine or b) by hydrogen halide hydrolysis of methoxypyrrolo[1,2-a]pyrazines and methoxypyrrolo[1,2-b]pyridazines. Protonation studies and some reactivity of the systems are discussed.DOI:10.3987/com-92-6085
-
作为产物:描述:参考文献:名称:Potent Odorants of Raw Arabica Coffee. Their Changes during Roasting摘要:Aroma extract dilution analysis of raw Arabica coffee revealed 3-isobutyl-2-methoxypyrazine (I), 2-methexy-3,5-dimethylpyrazine (II), ethyl 2-methylbutyrate (III), ethyl 3-methylbutyrate (IV), and 3-isopropyl-2-methoxypyrazine (V) as potent odorants. The highest odor activity value was found for I followed by II, IV, and V. It was concluded that I was responsible for the characteristic, peasy odor note of raw coffee. Twelve odorants occurring in raw coffee and (E)-beta-damascenone were also quantified after roasting. The concentration of I did not change, whereas methional, 3-hydroxy-4,5-direethyl-2(5H)-furanone, vanillin, (E)-beta-damascenone, and 4-vinyl- and 4-ethylguaiacol increased strongly during the roasting process.DOI:10.1021/jf990609n
文献信息
-
[EN] TRIAZOLE AGONISTS OF THE APJ RECEPTOR<br/>[FR] TRIAZOLES AGONISTES DU RÉCEPTEUR APJ申请人:AMGEN INC公开号:WO2016187308A1公开(公告)日:2016-11-24Compounds of Formula I and Formula II, pharmaceutically acceptable salt thereof, stereoisomers of any of the foregoing, or mixtures thereof are agonists of the APJ Receptor and have use in treating cardiovascular and other conditions. Compounds of Formula I and Formula II have the following structures where the definitions of the variables are provided herein.公式I和公式II的化合物,其药用盐,上述任何一种的立体异构体,或它们的混合物是APJ受体的激动剂,并可用于治疗心血管和其他疾病。公式I和公式II的化合物具有以下结构,其中变量的定义在此提供。
-
[EN] TRIAZOLE PYRIDYL COMPOUNDS AS AGONISTS OF THE APJ RECEPTOR<br/>[FR] COMPOSÉS DE TRIAZOLE PYRIDYLE EN TANT QU'AGONISTES DU RÉCEPTEUR APJ申请人:AMGEN INC公开号:WO2018093580A1公开(公告)日:2018-05-24Compounds of Formula I and Formula II, pharmaceutically acceptable salt thereof, stereoisomers of any of the foregoing, or mixtures thereof are agonists of the APJ Receptor and may have use in treating cardiovascular and other conditions. Compounds of Formula I and Formula II have the structures where the definitions of the variables are provided herein.化合物I和化合物II,其药用可接受盐,上述任何的立体异构体,或它们的混合物是APJ受体的激动剂,可能用于治疗心血管和其他疾病。化合物I和化合物II的结构如下,其中变量的定义在此处提供。
-
8-AZABICYCLO[3.2.1]OCTANE-8-CARBOXAMIDE DERIVATIVE申请人:Horiuchi Yoshihiro公开号:US20120225876A1公开(公告)日:2012-09-06Disclosed is a compound represented by formula (1) or a pharmacologically acceptable salt thereof (In the formula, A represents a group that is represented by formula (A-1); R 1a and R 1b may be the same or different and each independently represents a C 1-6 alkyl group which may be substituted by one to three halogen atoms; m and n each independently represents an integer of 0-5; X 1 represents a hydroxyl group or an aminocarbonyl group; Z 1 represents a single bond or the like; and R 2 represents an optionally substituted C 1-6 alkyl group, an optionally substituted C 6-10 aryl group or the like.)公开了一种由公式(1)表示的化合物或其药理可接受的盐(在公式中,A代表由公式(A-1)表示的基团;R1a和R1b可以相同或不同,每个独立地表示一个可以由一个到三个卤素原子取代的C1-6烷基;m和n各自独立地表示0-5之间的整数;X1代表羟基或氨基甲酰基;Z1代表单键等;R2代表一个可选地取代的C1-6烷基,一个可选地取代的C6-10芳基等)。
-
Tricyclic azaindolizine derivatives having an sPLA2-inhibitory activities申请人:Shionogi & Co., Ltd.公开号:US06756376B1公开(公告)日:2004-06-29The present invention provides a compound having sPLA2 inhibiting activity. The compound represented by the formula (I): wherein E is N or C—R4; G is N or C—R25; R1 is a carbocyclic group, heterocyclic group or the like; one of R3 and R4 is —(L2)-(acidic group), the other is a hydrogen atom, wherein L2 is a group connecting with an acid group; A ring is optionally substituted 5-8 membered cyclohexane ring or cyclohexene ring; R24 and R25 are a hydrogen atom or the like, its prodrug, their pharmaceutically acceptable salt, or hydrate thereof.
-
TRIAZOLE AGONISTS OF THE APJ RECEPTOR申请人:AMGEN INC.公开号:US20170044131A1公开(公告)日:2017-02-16Compounds of Formula I and Formula II, pharmaceutically acceptable salt thereof, stereoisomers of any of the foregoing, or mixtures thereof are agonists of the APJ Receptor and have use in treating cardiovascular and other conditions. Compounds of Formula I and Formula II have the following structures: where the definitions of the variables are provided herein.公式I和公式II的化合物,其药用可接受的盐,任何上述的立体异构体,或其混合物均为APJ受体激动剂,并可用于治疗心血管和其他疾病。公式I和公式II的化合物具有以下结构:其中变量的定义在此处提供。
表征谱图
-
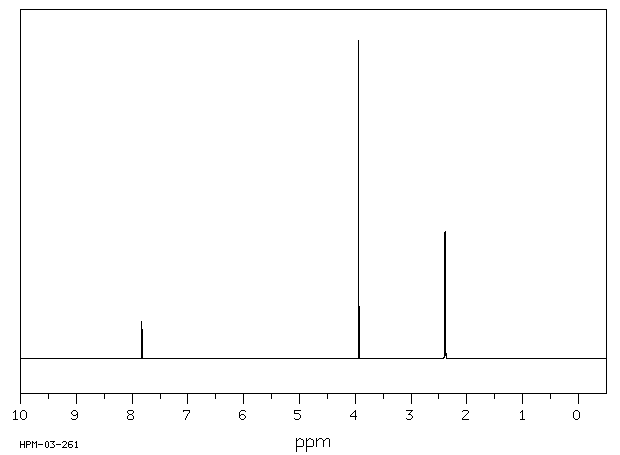
氢谱1HNMR
-
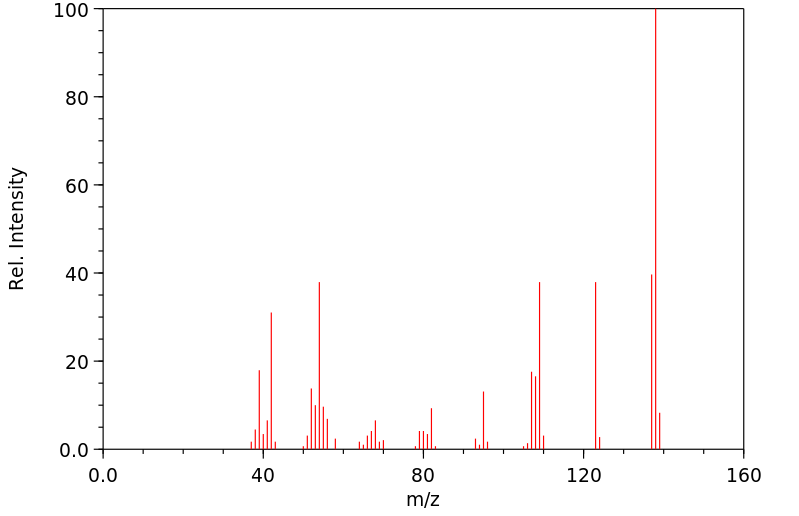
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3