4,6-二氯-2-甲基硫代-5-苯基嘧啶 | 64415-11-8
中文名称
4,6-二氯-2-甲基硫代-5-苯基嘧啶
中文别名
4,6-二氯-2-甲基硫代-5-苯基吡啶;4,6-二氯-2-甲基硫醇-5-苯基吡啶
英文名称
4,6-dichloro-2-methylthio-5-phenylpyrimidine
英文别名
4,6-dichloro-2-(methylthio)-5-phenylpyrimidine;4,6-Dichloro-5-phenyl-2-methylthiopyrimidine;4,6-dichloro-2-methylsulfanyl-5-phenyl-pyrimidine;4,6-dichloro-5-phenyl-2-methylthio pyrimidine;4,6-Dichlor-2-methylmercapto-5-phenyl-pyrimidin;4,6-dichloro-2-methylsulfanyl-5-phenylpyrimidine
CAS
64415-11-8
化学式
C11H8Cl2N2S
mdl
MFCD00006084
分子量
271.17
InChiKey
LMTMZMBTPXASSW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:107-111 °C(lit.)
-
沸点:364.3±42.0 °C(Predicted)
-
密度:1.4658 (rough estimate)
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):4.6
-
重原子数:16
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:51.1
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:8
-
安全说明:S26,S27,S28,S36/37/39,S45
-
危险类别码:R20/21/22
-
包装等级:III
-
WGK Germany:3
-
危险标志:GHS05,GHS07
-
危险品运输编号:UN 3263 8/PG 2
-
危险性描述:H314,H335
-
危险性防范说明:P261,P280,P305 + P351 + P338,P310
SDS
| Name: | 4 6-Dichloro-2-methylthio-5-phenylpyrimidine 95% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 64415-11-8 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 64415-11-8 | 4,6-Dichloro-2-methylthio-5-phenylpyri | 95 | 264-877-3 |
Risk Phrases: 20/21/22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.Lachrymator (substance which increases the flow of tears).Hygroscopic (absorbs moisture from the air).
Potential Health Effects
Eye:
Contact produces irritation, tearing, and burning pain.
Lachrymator (substance which increases the flow of tears). May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation:
Causes respiratory tract irritation. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Do NOT allow victim to rub eyes or keep eyes closed.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Will burn if involved in a fire.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Store protected from moisture.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 64415-11-8: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: dark yellow - off-white powder
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 107.00 - 111.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C11H8Cl2N2S
Molecular Weight: 271.15
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, exposure to moist air or water.
Incompatibilities with Other Materials:
Moisture, strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, oxides of sulfur, irritating and toxic fumes and gases, carbon dioxide, chlorine, chloride fumes.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 64415-11-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
4,6-Dichloro-2-methylthio-5-phenylpyrimidine - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TEAR GAS SUBSTANCE, SOLID, N.O.S.*
Hazard Class: 6.1
UN Number: 1693
Packing Group: II
IMO
Shipping Name: TEAR GAS SUBSTANCE, SOLID, N.O.S.
Hazard Class: 6.1
UN Number: 1693
Packing Group: II
RID/ADR
Shipping Name: TEAR GAS SUBSTANCE, SOLID, N.O.S.
Hazard Class: 6.1
UN Number: 1693
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
Safety Phrases:
WGK (Water Danger/Protection)
CAS# 64415-11-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 64415-11-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 64415-11-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4,6-二氯-5-苯基嘧啶 4,6-dichloro-5-phenylpyrimidine 3974-16-1 C10H6Cl2N2 225.077 —— 4-chloro-6-(4-chlorophenyl)-2-(methylthio)-5-phenylpyrimidine 917396-12-4 C17H12Cl2N2S 347.268
反应信息
-
作为反应物:描述:4,6-二氯-2-甲基硫代-5-苯基嘧啶 在 四(三苯基膦)钯 、 potassium hydroxide 作用下, 以 四氢呋喃 、 甲苯 为溶剂, 反应 15.0h, 生成 (3R,3'R,4S,4'S,6R,6'R)-6,6'-((1S,1'S)-((2-(naphthalen-1-yl)-5-phenylpyrimidine-4,6-diyl)bis(oxy))bis((6-methoxyquinolin-4-yl)methylene))bis(3-vinylquinuclidine)参考文献:名称:Catalytic, Asymmetric, Interrupted Feist–Bénary Reactions of α-Tosyloxyacetophenones摘要:A new variant of the Interrupted Feist-Benary (IFB) reaction uses alpha-tosyloxyacetophenones as electrophiles and proceeds in good yields and excellent enantioselectivities.DOI:10.1021/ol2026697
-
作为产物:描述:参考文献:名称:2-(甲基磺酰基)嘧啶衍生物的便捷合成摘要:摘要 通过丙二酸衍生物与 S-甲基异硫脲硫酸盐的循环缩合,然后在水-丙酮混合物中使用 oxone 作为氧化剂进行衍生和氧化,开发了一种有效且方便的制备功能化 2-(甲基磺酰基)嘧啶衍生物的方法。这种合成策略提供了一种高效且环保的方法,可以轻松获得具有可观产率的 2-(甲基磺酰基)嘧啶衍生物。图形概要DOI:10.1080/00397911.2017.1421664
文献信息
-
Triazolopyrimidine cannabinoid receptor 1 antagonists申请人:Wu Gang公开号:US20060287341A1公开(公告)日:2006-12-21The present application describes compounds according to both Formulas I and II, pharmaceutical compositions comprising at least one compound according to either Formula I or II and optionally one or more additional therapeutic agents, and methods of treatment using the compounds according to Formulas I and II both alone and in combination with one or more additional therapeutic agents. The compounds have the following general formulas: including all prodrugs, solvates, pharmaceutically acceptable salts and stereoisomers, wherein R 1 , R 2 , R 3 , R 4 and R 5 are described herein.本申请描述了根据公式I和II的化合物,包括至少一种符合公式I或II的化合物的药物组合物,以及可选地包括一种或多种额外治疗剂的药物组合物,并且使用根据公式I和II的化合物进行治疗的方法,无论是单独使用还是与一种或多种额外治疗剂结合使用。这些化合物具有以下一般公式:包括所有的前药、溶剂化合物、药用可接受盐和立体异构体,其中R1、R2、R3、R4和R5如本文所述。
-
[1,2,4] triazolo [1,5-C] pyrimidine derivatives申请人:Kyowa Hakko Kogyo Co., Ltd.公开号:US06222035B1公开(公告)日:2001-04-24[1,2,4]Triazolo[1,5-c]pyrimidine derivatives represented by formula (I) or pharmaceutically acceptable salts thereof are provided, which have adenosine A2A receptor antagonism and are useful for the treatment or prevention of various diseases induced by hyperactivity of adenosine A2A receptors (for example, Parkinson's disease or senile dementia): wherein R1 represents substituted or unsubstituted aryl, or the like; R2 represents hydrogen, halogen, lower alkyl, substituted or unsubstituted aryl, or the like; R3 represents hydrogen, halogen, XR10 (wherein X represents O or S; and R10 represents substituted or unsubstituted aryl, a substituted or unsubstituted aromatic heterocyclic ring, substituted or unsubstituted aralkyl, lower alkyl, or hydroxy lower alkyl), or the like; and Q represents hydrogen or 3,4-dimethoxybenzyl.
-
Triazolo [4,3-c]pyrimidines substituted by nitrogen-containing申请人:Riker Laboratories, Inc.公开号:US04477450A1公开(公告)日:1984-10-161,2,4-Triazolo[4,3-c]pyrimidines substituted at the 5 or 7 position through a nitrogen atom which is part of a heterocyclic ring have been found to have potent bronchodilator activity and to be useful synthetic intermediates in the preparation of 1,2,4-triazolo[1,5-c]-pyrimidines. Methods for inducing bronchodilation, pharmaceutical compositions, and synthetic processes and intermediates are also described.
-
Pyrimidines substituted by nitrogen-containing heterocyclic rings as申请人:Riker Laboratories, Inc.公开号:US04536579A1公开(公告)日:1985-08-201,2,4-Triazolo[4,3-c]pyrimidines substituted at the 5 or 7 position through a nitrogen atom which is part of a heterocyclic ring have been found to have potent bronchodilator activity and to be useful synthetic intermediates in the preparation of 1,2,4-triazolo[1,5-c]pyrimidines. Methods for inducing bronchodilation, pharmaceutical compositions, and synthetic processes and intermediates are also described.
-
INDOL DERIVATIVES USEFUL FOR THE TREATMENT OF MIGRAINE, COMPOSITION AND UTILIZATION申请人:VITA-INVEST, S.A.公开号:EP0714896A1公开(公告)日:1996-06-05Indole derivatives having the Formula (I) wherein R₁ is H, alkyl or acyl group; R₂ is H, halogen or alkyl, hydroxy, alkoxy; ciano, carboxamide, carboxyl, alkoxicarbonyl, phenyl, phenyloxy or a group -(CH₂)n-R₆, (n=1-3), and R₆ is a group ciano, nitro, phenyl, carboxyl, -CO₂R₇; -CONR₇R₈; -SO₂NR₇R₈; -COR₇; -SO₂R₇, R₇ and R₈ being H or alkyl; R₇ and R₈, together with N, can form a cycle of 4-7 links; R₃, R₄ and R₅ are H, halogen or a group alkyl, phenyl, substituted phenyl, hydroxy, alcoxy, phenyloxy or benziloxy; NR₁₁R₁₂ is H or a group ciano, nitro, carboxyl, alcoxycarbonyl, carboxamido or a group R₃; A is an alkyliden group -(CH₂)-m, (m = 1-5), or alkenyl group; B is a piperazinoring (a), aminopyrrolidinoring (b); pyrrolidinamino ring (c); piperidinoring (d) or ethylendiamino -NR₉-CH₂-CH₂-NR₁₀-, R₉ and R₁₀ being H or an alkyl group. The compounds are useful for the treatment of migraine.吲哚衍生物具有以下式(I)其中R₁为H、烷基或酰基;R₂为H、卤素或烷基、羟基、烷氧基;氰基、羧酰胺基、羧基、烷氧基羰基、苯基、苯氧基或一个基团-(CH₂)n-R₆,(n=1-3),且R₆为一个基团氰基、硝基、苯基、羧基、-CO₂R₇;-CONR₇R₈;-SO₂NR₇R₈;-COR₇;-SO₂R₇,R₇和R₈为H或烷基;R₇和R₈,与N一起,可形成一个4-7个连接的环;R₃、R₄和R₅为H、卤素或一个烷基、苯基、取代苯基、羟基、烷氧基、苯氧基或苄氧基基团;NR₁₁R₁₂为H或一个基团氰基、硝基、羧基、烷氧羰基、羧酰胺基或一个基团R₃;A为一个烷基亚甲基基团-(CH₂)-m,(m=1-5),或烯基基团;B为哌嗪环(a)、氨基吡咯啉环(b);吡咯啉氨基环(c);哌啶环(d)或乙二胺-NR₉-CH₂-CH₂-NR₁₀-,R₉和R₁₀为H或一个烷基基团。这些化合物可用于治疗偏头痛。
表征谱图
-
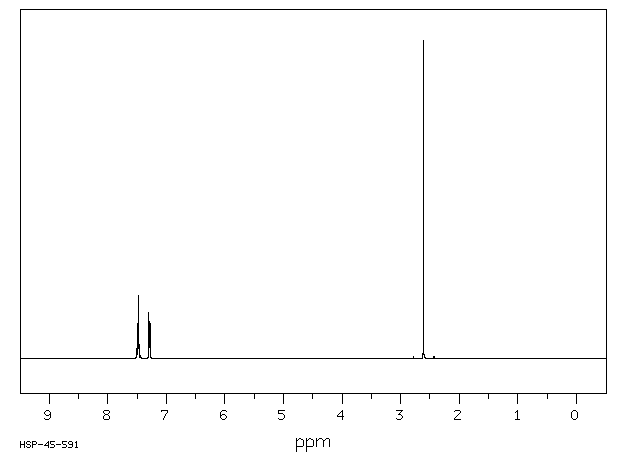
氢谱1HNMR
-
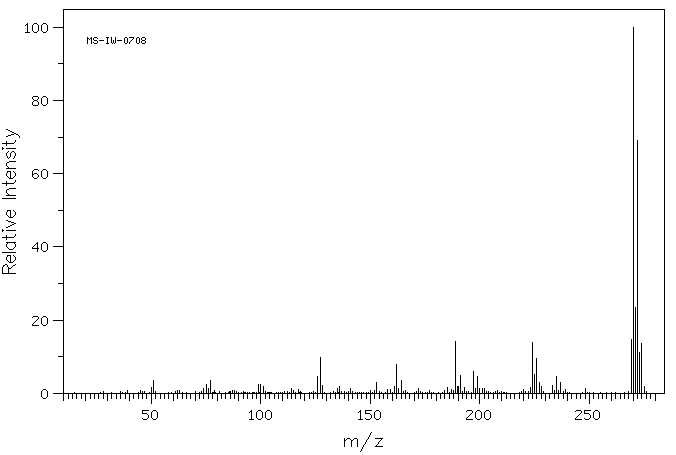
质谱MS
-
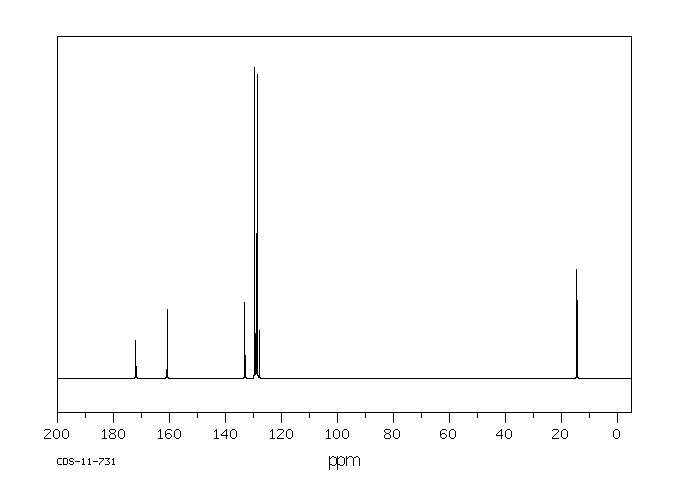
碳谱13CNMR
-
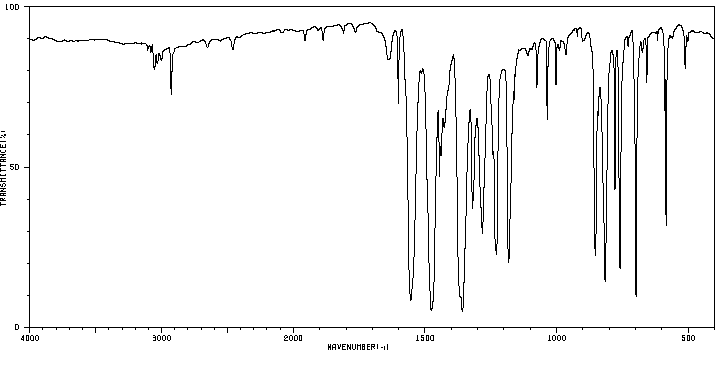
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
颜料红88
颜料紫36
顺式-1,2-二(乙硫基)-1-丙烯
非班太尔-D6
雷西那得中间体
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛-1,7-二炔-1-基(苯基)硫烷
西嗪草酮
萘,2-[(2,3-二甲基苯基)硫代]-
莫他哌那非
茴香硫醚
苯醌B
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-甲基苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2,6-二氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,2-[(2-硝基苯基)硫代]-
苯酚,3-氯-4-[(4-硝基苯基)硫代]-
苯酚,3-(乙硫基)-
苯酚,3,5-二[(苯基硫代)甲基]-
苯胺,4-[5-溴-3-[4-(甲硫基)苯基]-2-噻嗯基]-
苯胺,3-氯-4-[(1-甲基-1H-咪唑-2-基)硫代]-
苯胺,2-[(2-吡啶基甲基)硫代]-
苯硫醚-D10
苯硫胍
苯硫基乙酸
苯硫代磺酸S-(三氯乙烯基)酯
苯甲醇,2,3,4,5,6-五氟-a-[(苯基硫代)甲基]-,(R)-
苯甲酸,3-[[2-[(二甲氨基)甲基]苯基]硫代]-,盐酸
苯甲胺,5-氟-2-((3-甲氧苯基)硫代)-N,N-二甲基-,盐酸
苯甲二硫酸,4-溴苯基酯