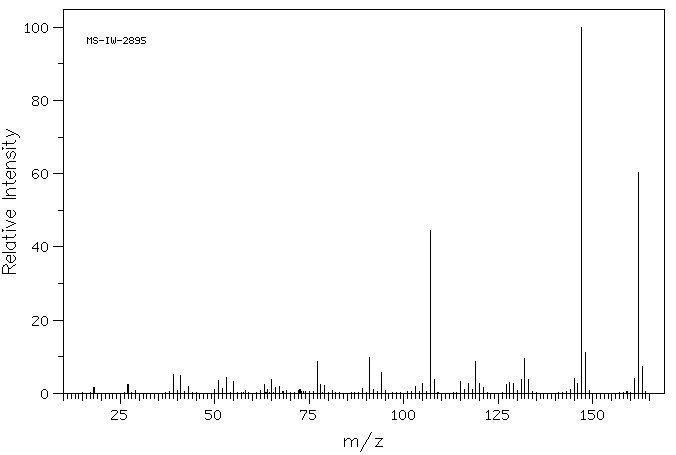
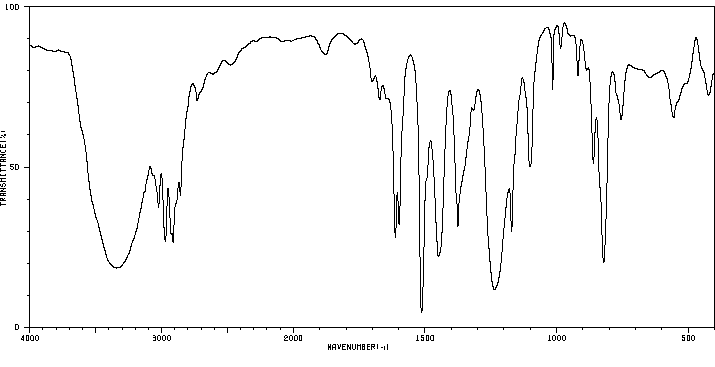
4-(3-甲基丁-2-烯基)苯酚 | 1200-09-5
中文名称
4-(3-甲基丁-2-烯基)苯酚
中文别名
4-(3-甲基-2-丁烯基)苯酚
英文名称
4-(3-methylbut-2-en-1-yl)phenol
英文别名
4-(3-methyl-2-butenyl)phenol;4-(3-methylbut-2-enyl)phenol;4-(3,3-dimethylallyl)phenol;p-prenyl phenol;4-prenylphenol;p-prenylphenol;PHENOL, p-(3-METHYL-2-BUTENYL)-
CAS
1200-09-5
化学式
C11H14O
mdl
——
分子量
162.232
InChiKey
CBUZIDRVPSPPOE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
存在于主流烟气中。
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.27
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : Phenol, p-(3-methyl-2-butenyl)-
CAS-No. : 1200-09-5
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No 1272/2008
This substance is not classified as dangerous according to Directive 67/548/EEC.
Label elements
Caution - substance not yet tested completely.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C11H14O
Molecular Weight : 162,22 g/mol
Section 4. FIRST AID MEASURES
Description of first aid measures
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration.
In case of skin contact
Wash off with soap and plenty of water.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water.
Most important symptoms and effects, both acute and delayed
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Avoid dust formation. Avoid breathing vapors, mist or gas.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Sweep up and shovel. Keep in suitable, closed containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
General industrial hygiene practice.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Choose body protection in relation to its type, to the concentration and amount of dangerous
substances, and to the specific work-place., The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection is not required. Where protection from nuisance levels of dusts are desired,
use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes May cause eye irritation.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-氯-4-(3-甲基丁-2-烯基)苯 1-chloro-4-(3-methyl-2-butenyl)benzene 23853-76-1 C11H13Cl 180.677 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-methoxy-4-(3-methyl-but-2-enyl)benzene 4957-18-0 C12H16O 176.258 —— 4-(3-methyl-3-butenyl)phenol 18272-69-0 C11H14O 162.232 4-异戊基苯酚 p-isoamylphenol 1805-61-4 C11H16O 164.247 —— 2,6-bis-hydroxymethyl-4-(3-methyl-but-2-enyl)-phenol 116632-93-0 C13H18O3 222.284
反应信息
-
作为反应物:描述:参考文献:名称:Unsaturated Phenols. V. The Reaction of Isoprene with Phenol1摘要:DOI:10.1021/ja01545a043
-
作为产物:描述:[(3-甲基-2-丁烯-1-基)氧基]苯 以 N,N-二甲基苯胺 为溶剂, 反应 1.0h, 以65%的产率得到4-(3-甲基丁-2-烯基)苯酚参考文献:名称:烯丙基化和异戊二烯化铬酮的串联克莱森重排/6-内环化方法摘要:源自邻酰基苯酚的烯丙基、二甲基烯丙基和异戊二烯醚在微波辐射下反应形成 C-烯丙基化或异戊二烯化色酮衍生物,具体取决于芳烃和烯丙基取代基的取代模式。该反应通过串联克莱森重排和 6-endo-trig 或 6-endo-dig 环化序列进行。对于异戊二烯醚,串联序列可以通过 Cope 重排扩展以提供 6-异戊二烯色酮。该方法可用于合成天然产物和药物。DOI:10.1002/ejoc.201501151
文献信息
-
Clay catalyzed rearrangement of substituted allyl phenyl ethers: Synthesis of ortho-allyl phenols, chromans and coumarans作者:William G. Dauben、Jeffrey M. Cogen、Victor BeharDOI:10.1016/s0040-4039(00)89033-4日期:1990.1Montmorillonite clays catalyze the rearrangement of substituted allyl phenyl ethers to provide ortho-allyl phenols, chromans and coumarans under mild conditions.
-
Palladium-Catalyzed Completely Linear-Selective Negishi Cross-Coupling of Allylzinc Halides with Aryl and Vinyl Electrophiles作者:Yang Yang、Thomas J. L. Mustard、Paul Ha-Yeon Cheong、Stephen L. BuchwaldDOI:10.1002/anie.201308585日期:2013.12.23Completely linear: The title reaction provides an effective means to access a wide range of prenylated arenes and “skipped dienes” in a completely linear‐selective fashion, as demonstrated by a concise synthesis of the anti‐HIV natural product siamenol. DFT calculations shed light on the origin of the excellent regioselectivity observed with the current Pd‐based catalyst system.
-
Effective Synthesis of 2-[4-(3-Methyl-2-butenyl)phenyl]propionic Acid via Hydromagnesation Reaction with Nickel or Titanium Catalysts作者:Takehiro Amano、Tomomi Ota、Kensei Yoshikawa、Tatsuhiko Sano、Yutaka Ohuchi、Fumie Sato、Manzo Shiono、Yoshiji FujitaDOI:10.1246/bcsj.59.1656日期:1986.52-[4-(3-Methyl-2-butenyl)phenyl]propionic acid was synthesized in 82% yield via nickel(II) chloride or dichlorobis[η5-cyclopentadienyl]titanium-catalyzed hydromagnesation of 1-(3-methyl-2-butenyl)-4-vinylbenzene obtained in ca. 63–68% yield by the coupling reaction of 4-(3-methyl-2-butenyl)phenylmagnesium chloride with vinyl chloride.
-
Magnesium dicarboxylates promote the prenylation of phenolics that is extended to the total synthesis of icaritin作者:Jichao Zhang、Wei Xiong、Yongju Wen、Xuewen Fu、Xiaoxia Lu、Guolin Zhang、Chun WangDOI:10.1039/d1ob02228h日期:——study of the prenylation provided evidence for the nucleophilic addition/substitution of the phenolic substrate to the alkyl halide in the presence of the magnesium dicarboxylates. The proto application of this method in the total synthesis of icaritin through the prenylation of 2,4,6-trihydroxyacetophenone, followed by the reaction with benzaldehyde to afford the flavonol, was successful, with a total开发了由二羧酸镁促进的酚类底物的异戊烯化。对该范围的调查表明,具有给电子基团的底物比具有吸电子基团的底物产生更好的产率。尽管所有底物在 MeCN 中的转化率都高于 DMF,但 DMF 仍然是多酚底物的有利溶剂,因为 MeCN 会导致生成环化副产物 ( 6 ) 并降低3的收率。对于那些对位未占据的底物,邻位与对位异戊二烯化(3'与3'' )的区域选择性也与溶剂有关。DMF主要生产邻位-产品,但转化率低。另一方面,MeCN 主要产生对位产物,以及次要的邻位产物。异戊二烯化的机理研究为在二羧酸镁存在下酚底物亲核加成/取代到卤代烷提供了证据。该方法在2,4,6-三羟基苯乙酮异戊烯化后与苯甲醛反应得到黄酮醇的全合成淫羊藿苷中的初步应用是成功的,总收率为33%。
-
Montmorillonite Clays in Organic Synthesis: A One-Pot Conversion of Phenols to 2,2-Dimethylbenzopyrans作者:Matthew Dintzner、Kristen McClelland、Kara Morse、Michael AkroushDOI:10.1055/s-2004-830865日期:——2,2-Dimethylbenzopyran derivatives were generated through a one-pot Montmorillonite K10 clay-catalyzed condensation of substituted phenols with prenyl bromide.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚