1,2-Benzo-dinaphtho-<2',3':4,5>-<2'',3'':8,9>-pyren | 192-46-1
中文名称
——
中文别名
——
英文名称
1,2-Benzo-dinaphtho-<2',3':4,5>-<2'',3'':8,9>-pyren
英文别名
Dibenzoheptaphen;1,2-Benzo-dinaphtho-[2',3':4,5]-[2'',3'':8,9]-pyren;Dibenzo(J,xyz)heptaphene;nonacyclo[18.14.2.02,11.04,9.013,35.014,19.022,31.024,29.032,36]hexatriaconta-1(35),2,4,6,8,10,12,14,16,18,20,22,24,26,28,30,32(36),33-octadecaene
CAS
192-46-1
化学式
C36H20
mdl
——
分子量
452.555
InChiKey
KADSIADUIOVQLQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):10.2
-
重原子数:36
-
可旋转键数:0
-
环数:9.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
反应信息
-
作为产物:描述:4,5-Benzodinaphtho<2'.3':1,2><2'',3'':7,8>pyren-1',4':1'',4''-dichinon 在 sodium chloride 、 zinc(II) chloride 、 锌 作用下, 生成 1,2-Benzo-dinaphtho-<2',3':4,5>-<2'',3'':8,9>-pyren参考文献:名称:9,10-蒽醌二甲烷和9-亚甲基蒽酮的狄尔斯-阿尔德反应摘要:研究了9,10-蒽醌二甲烷与马来酸酐,乙炔二羧酸二甲酯,对苯醌和1,4-萘醌的加成反应。报道了苯并[ a ] py ,二苯并[ j,xyz ]-庚苯以及这些烃的一些取代和还原衍生物的合成,并制备了1,4:11,14-二苯并[ h,rst ]五苯二醌。研究了10-羟基-10-甲基蒽酮与乙炔二羧酸二甲酯和偶氮二羧酸二乙酯的反应。后者给出了新颖的7 H-二-苯并[ de,h ]邻苯二嗪体系。DOI:10.1039/j39670000855
文献信息
-
Higher annelated pyrenes—II作者:E. Clar、M. ZanderDOI:10.1016/s0040-4020(01)98541-2日期:1963.15]-pyrene (II), 1,2-benzo-naphtho-2′,3′:6,7]-pyrene (III) and 1,2-benzo-dinaphtho-[2′,3′:4,5]; [2″,3″ :8,9] pyrene (VI) were obtained by pyrolysis of the ketone (I). The hydrocarbon (II) was also prepared by a synthesis with phthalic anhydride. Pyrolysis of the ketone (VIII) yields 3,4-benzo-naphtho-[2′,3′:9,10]-pyrene (VIII).
表征谱图
-
氢谱1HNMR
-
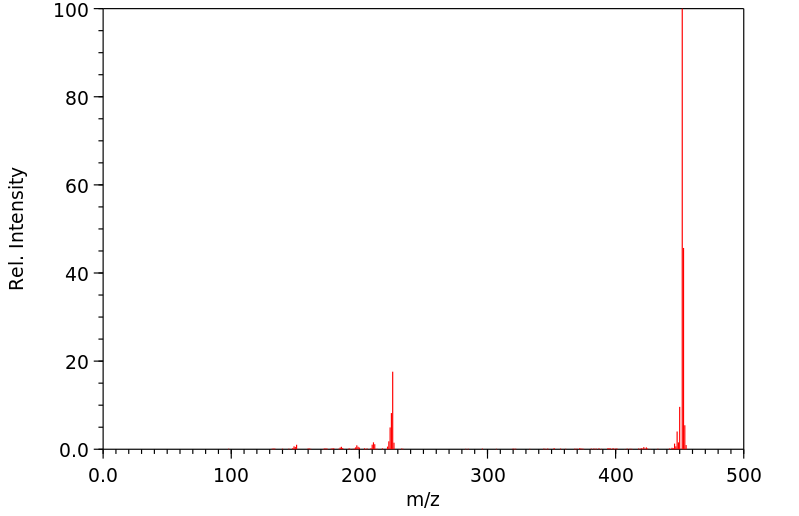
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-1,2-二(1-芘基)环丁烷
顺式-1,2-二(1-芘基)环丁烷
顺式-(-)-苯并(a)芘-7,8-二醇-9,10-环氧化物
雄甾烷
还原黑29
还原黄4
还原金橙G
还原绿2
还原绿1
还原紫3B
还原紫 10
还原深蓝BO
还原橙4
还原橙2
还原兰黑BBN
还原亮橙IRK
试剂N1,N1,N3,N3,N6,N6,N8,N8-Octakis(4-methoxyphenyl)-1,3,6,8-pyrenetetramine
蒽酮紫79
蒽缔蒽酮
蒽并(1,2,3,4-ghi)苝
蒽嵌蒽
蒽[9,1,2-cde]苯并[rst]戊芬
萘并[2'.8',2.4]晕苯
萘并[2',1',8',7':4,10,5]蒽并[1,9,8-abcd]晕苯
萘并[1,8-gh:4,5-g'h']二喹啉
萘并(8,1,2-bcd)苝
萘并(2,3-a)晕苯
萘并(2,1,8-qra)萘并萘-7 12-二酮
萘并(1,2,3-mno)醋菲烯
萘[2,3-a]芘
菲并[1,10,9,8-opqra]苝
茚并(1,2,3-cd)芘
苯胺,2-氯-3-(苯基甲氧基)-
苯并[xyz]庚芬
苯并[wx]萘并[2,1,8,7-hijk]庚省
苯并[rst]菲并[1,10,9-cde]戊芬
苯并[rst]戊酚-5-甲醛
苯并[pqr]四苯-5-基甲酸根
苯并[pqr]四苯-11-基甲酸根
苯并[pqr]二萘并[8,1,2-bcd:2',1',8'-lmn]苝
苯并[p]萘并[1,8,7-ghi]屈
苯并[l]芘-8-醇
苯并[ghi]苝
苯并[e]芘
苯并[b]芘-6-基甲醇
苯并[b]芘-6,12-二酮
苯并[b]芘-3,6-二酮
苯并[b]芘-1,6-二酮
苯并[a]芘-9,10-环氧化物
苯并[a]芘-7-醇