4-(p-硝基苯氧基)丁酸甲酯 | 28341-53-9
中文名称
4-(p-硝基苯氧基)丁酸甲酯
中文别名
4-(对硝基苯氧基)-丁基甲酯
英文名称
methyl 4-(4-nitrophenoxy)butyrate
英文别名
4-(4-nitrophenoxy)-n-butyric acid methyl ester;p-Nitro-phenoxy-buttersaeure-methylester;Methyl 4-(p-nitrophenoxy)butyrate;methyl 4-(4-nitrophenoxy)butanoate
CAS
28341-53-9
化学式
C11H13NO5
mdl
MFCD00043608
分子量
239.228
InChiKey
MJMZBXYBWMBFNY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:56-57 °C(Solv: benzene (71-43-2); hexane (110-54-3))
-
沸点:377.1±22.0 °C(Predicted)
-
密度:1.228±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:17
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.363
-
拓扑面积:81.4
-
氢给体数:0
-
氢受体数:5
安全信息
-
海关编码:2918990090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 4-(4-nitrophenoxy)butanoic acid 28341-54-0 C10H11NO5 225.201 对硝基苯酚 4-nitro-phenol 100-02-7 C6H5NO3 139.111 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-(4-nitrophenoxy)butanoic acid 28341-54-0 C10H11NO5 225.201 —— 4-(4-nitro-phenoxy)-butyryl chloride 30312-88-0 C10H10ClNO4 243.647 —— Methyl 4-(4-aminophenoxy)butanoate 130198-73-1 C11H15NO3 209.245 —— 4-{4-[bis-(2-chloro-ethyl)-amino]-phenoxy}-butyric acid methyl ester 500895-96-5 C15H21Cl2NO3 334.243
反应信息
-
作为反应物:描述:参考文献:名称:바르비투르산 유도체 화합물을 유효성분으로 포함하는 살충제摘要:本发明提供了一种可以用于开发新的杀虫剂原料,以替代产生多种副作用,如抗性害虫的发展,对人畜的毒性,环境污染和残留问题等有机合成农药的巴比妥酸诱导体化合物,以及包含其作为有效成分的杀虫剂组合物。公开号:KR20220066701A
-
作为产物:描述:对硝基苯酚 、 4-溴丁酸甲酯 在 potassium carbonate 、 potassium iodide 作用下, 以 丙酮 为溶剂, 反应 72.0h, 以2.2 g的产率得到4-(p-硝基苯氧基)丁酸甲酯参考文献:名称:바르비투르산 유도체 화합물을 유효성분으로 포함하는 살충제摘要:本发明提供了一种可以用于开发新的杀虫剂原料,以替代产生多种副作用,如抗性害虫的发展,对人畜的毒性,环境污染和残留问题等有机合成农药的巴比妥酸诱导体化合物,以及包含其作为有效成分的杀虫剂组合物。公开号:KR20220066701A
文献信息
-
Production method of polyhydroxyalkanoate form substituted fatty acid ester as raw material申请人:——公开号:US20020081646A1公开(公告)日:2002-06-27Polyhydroxyalkanoates comprising a monomer unit having the following chemical formula (2): 1 (where the reference characters R represents an arbitrarily selected substituent and x represents an integer of 0 to 8) is produced by culturing a microorganism in a culture medium containing a substituted fatty acid ester having the following chemical formula (1): 2 (where the reference characters R and R′ separately denote an optional substituent and x represents an integer of 0 to 8). The microorganism is capable of taking the substituted fatty acid ester into the cells and synthesizing the desired polyhydroxyalkanoate in the culture medium.
-
Production method of polyhydroxyalkanoate from substituted fatty acid ester as raw material申请人:CANON KABUSHIKI KAISHA公开号:EP1201763A2公开(公告)日:2002-05-02Polyhydroxyalkanoates comprising a monomer unit having the following chemical formula (2): (where the reference characters R represents an arbitrarily selected substituent and x represents an integer of 0 to 8) is produced by culturing a microorganism in a culture medium containing a substituted fatty acid ester having the following chemical formula (1): (where the reference characters R and R' separately denote an optional substituent and x represents an integer of 0 to 8). The microorganism is capable of taking the substituted fatty acid ester into the cells and synthesizing the desired polyhydroxyalkanoate in the culture medium.
-
The discovery and optimization of novel dual inhibitors of topoisomerase ii and histone deacetylase作者:Xuan Zhang、Bin Bao、Xiuhua Yu、Linjiang Tong、Yu Luo、Qingqing Huang、Mingbo Su、Li Sheng、Jia Li、Hong Zhu、Bo Yang、Xiongwen Zhang、Yi Chen、Wei LuDOI:10.1016/j.bmc.2013.09.023日期:2013.11A novel class of podophyllotoxin derivatives have been designed and synthesized based on the synergistic antitumor effects of topoisomerase II and histone deacetylase inhibitors. Their inhibitory activities towards histone deacetylases and Topo II and their cytotoxicities in cancer cell lines were evaluated. The aromatic capping group connection, linker length and zinc-binding group were systematically varied and preliminary conclusions regarding structure-activity relationships are discussed. Among all of the synthesized hybrid compounds, compound 24d showed the most potent HDAC inhibitory activity at a low nanomolar level and exhibited powerful antiproliferative activity towards HCT116 colon carcinoma cells at a low micromolar level. Further exploration of this series led to the discovery of potent dual inhibitor 32, which exhibited the strongest in vitro cytotoxic activity. (C) 2013 Elsevier Ltd. All rights reserved.
-
DNA-directed alkylating agents. 3. Structure-activity relationships for acridine-linked aniline mustards: consequences of varying the length of the linker chain作者:Kisione K. Valu、Trudi A. Gourdie、Theodore J. Boritzki、G. Lance Gravatt、Bruce C. Baguley、William R. Wilson、Laurence P. G. Wakelin、Paul D. Woodgate、William A. DennyDOI:10.1021/jm00173a016日期:1990.11Four series of acridine-linked aniline mustards have been prepared and evaluated for in vitro cytotoxicity, in vivo antitumor activity, and DNA cross-linking ability. The anilines were attached to the DNA-intercalating acridine chromophores by link groups (-O-, -CH2-, -S-, and -SO2-) of widely varying electronic properties, providing four series of widely differing mustard reactivity where the alkyl chain linking the acridine and mustard moieties was varied from two to five carbons. Relationships were sought between chain length and biological properties. Within each series, increasing the chain length did not alter the reactivity of the alkylating moiety but did appear to position it differently on the DNA, since cross-linking ability (measured by agarose gel assay) altered with chain length, being maximal with the C4 analogue. The in vivo antitumor activities of the compounds depended to some extent on the reactivity of the mustard, with the least reactive SO2 compounds being inactive. However, DNA-targeting did appear to allow the use of less reactive mustards, since the S-linked acridine mustards showed significant activity whereas the parent S-mustard did not. Within each active series, the most active compound was the C4 homologue, suggesting some relationship between activity and extent of DNA alkylation.
-
VALU, KISIONE K.;GOURDIE, TRUDI A.;BORITZKI, THEODORE J.;GRAVATT, G. LANC+, J. MED. CHEM., 33,(1990) N1, C. 3014-3019作者:VALU, KISIONE K.、GOURDIE, TRUDI A.、BORITZKI, THEODORE J.、GRAVATT, G. LANC+DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
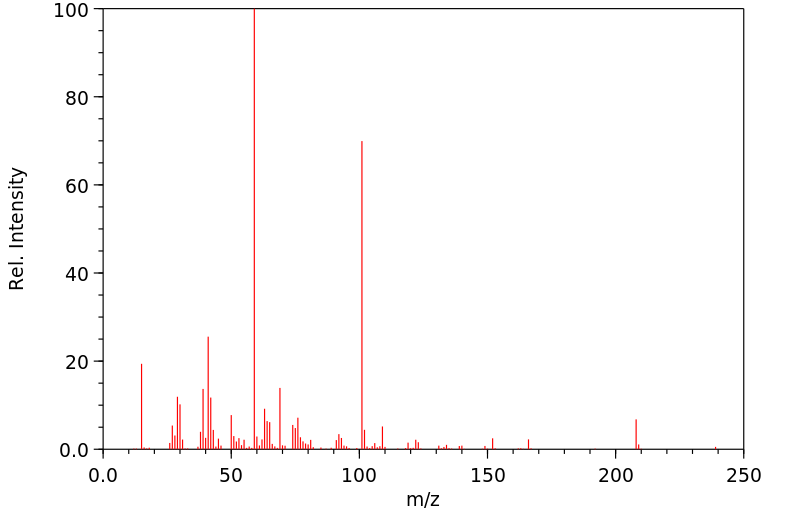
质谱MS
-
碳谱13CNMR
-
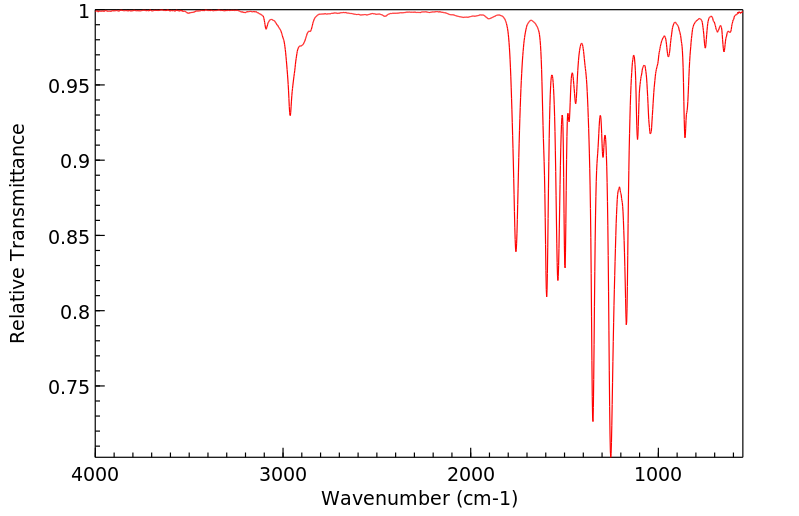
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫