4-戊氧基苯二甲腈 | 106943-83-3
中文名称
4-戊氧基苯二甲腈
中文别名
1,2-二氰基-4-戊氧基苯;4-戊氧基酞腈;4-正戊氧基邻苯二甲腈
英文名称
1,2-dicyano-4-(pentyloxy)benzene
英文别名
4-pentyloxyphthalonitrile;4-pentoxyphthalonitrile;4-pentoxybenzene-1,2-dicarbonitrile
CAS
106943-83-3
化学式
C13H14N2O
mdl
MFCD00059103
分子量
214.267
InChiKey
LEYRHJFOAFEIDH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:35 °C
-
沸点:387.9±32.0 °C(Predicted)
-
密度:1.08±0.1 g/cm3(Predicted)
-
溶解度:溶于甲醇
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:16
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.384
-
拓扑面积:56.8
-
氢给体数:0
-
氢受体数:3
反应信息
-
作为反应物:描述:4-戊氧基苯二甲腈 在 zinc diacetate 、 1,8-二氮杂双环[5.4.0]十一碳-7-烯 作用下, 反应 2.0h, 以72%的产率得到[2(3),9(10),16(17),23(24)-tetrakis(pentyloxy)phthalocyaninato]zinc(II)参考文献:名称:Efficient Synthesis of Transition-Metal Phthalocyanines in Functional Ionic Liquids摘要:报道了在1,8-二氮杂双环[5.4.0]十一碳-7-烯(DBU)和金属盐的存在下,以功能化咪唑鎓、吡啶鎓和铵离子液体为介质,在100-140 °C下,通过取代和不取代的邻苯二甲腈反应合成过渡金属酞菁的方法。在丁基(2-羟基乙基)二甲基溴化铵离子液体中,得到了金属酞菁的最佳产率。在上述离子液体中,利用不同的金属盐对自由基酞菁进行金属化,也获得了良好的产率。DOI:10.1055/s-2007-990879
文献信息
-
Photophysical and electrochemical properties of 1,3-bis(2-pyridylimino)isoindolate platinum(ii) derivatives作者:Kenneth Hanson、Luke Roskop、Niral Patel、Laurent Griffe、Peter I. Djurovich、Mark S. Gordon、Mark E. ThompsonDOI:10.1039/c2dt30354j日期:——phosphorescence ranging in energy from 594 to 680 nm was observed from the complexes, with quantum efficiencies ranging from 0.01 to 0.05. The efficiency of emission is dictated largely by nonradiative processes since the rate constants for nonradiative deactivation [(1.1–100) × 105 s−1] show greater variation than those for radiative decay [(0.57–4.0) × 04 s−1]. Nonradiative deactivation for compounds已经合成并表征了一系列十二个形式为(N ^ N ^ N)PtX的铂(II)配合物,其中N ^ N ^ N是1,3-双(2-吡啶基氨基)异吲哚酸酯配体(BPI)或BPI配体其芳基部分被叔取代-丁基,硝基,烷氧基,碘或氯基,并且X是氯,氟,氰基,乙酸酯,苯基或4-(二甲基氨基)苯基配体。所有络合物在电势下显示至少一个不可逆的氧化和两个可逆的还原波,其电势取决于X和取代的N ^ N ^ N配体两者的位置和给电子或吸电子的性质。从配合物中观察到宽范围的室温磷光,能量范围为594至680 nm,量子效率范围为0.01至0.05。发射效率主要由非辐射过程决定,因为非辐射失活的速率常数[(1.1–100)×10 5 s -1 ]显示出比辐射衰变[(0.57–4.0)×0 4 s -1 ]大的变化率。]。X = Cl的化合物的非辐射失活遵循能隙定律,即,非辐射速率常数随发射能量的降低呈指数增长。激发态的失
-
Influence of Substituents, Reaction Conditions and Central Metals on the Isomer Distributions of 1(4)-Tetrasubstituted Phthalocyanines作者:Christine Rager、Gabriele Schmid、Michael HanackDOI:10.1002/(sici)1521-3765(19990104)5:1<280::aid-chem280>3.0.co;2-0日期:1999.1.4The 1(4)- and 2(3)-tetraalkoxy-substituted nickel (5), copper (6), and metal-free phthalocyanines 7 and 8 were synthesized from the corresponding substituted phthalonitriles 2 and 4, respectively, and the four structural isomers formed for each phthalocyanine were separated by HPLC. In the case of 1(4)-tetraalkoxy-substituted phthalocyanines the ratio of the four isomers was found to be different from the expected statistical distribution of D-2h:C-5:C-2v:C-4h = 12.5:50:25:12.5. For the 1(4)-substituted metal-fret: phthalocyanine 7 a very high proportion of the C-4h isomer (87%) is formed. In the case of the 1(4)-substituted metal phthalocyanines 5 and 6 the strikingly low proportion of the D-2h isomer (found: 2-4% compared to statistical distribution: 12.5%) is interpreted by a template mechanism (given in Scheme 2) in which strong steric hindrance of the respective neighboring groups prevent the formation of the D-2h isomer. To investigate further the mechanism of formation of phthalocyanines the synthesis of 1(4)- and 2(3)-tetraalkoxy-substituted metal phthalocyanines containing chiral alkoxy groups (13-17) was studied under different reaction conditions starting from the corresponding alkoxy-substituted phthalonitriles 10 and 11. In all investigated cases the chiral alkoxy groups in the starting phthalonitrile again affect the distribution of the structural isomers of the formed phthalocyanines, lending to a higher proportion of the C-4h isomer in comparison with the 1(4)-tetraalkoxy-substituted phthalocyanines with racemic alkoxy groups.
-
Recoverable polymer-bound homogeneous catalysts for catalytic chain transfer process申请人:Bryndza Henry Edward公开号:US20100261851A1公开(公告)日:2010-10-14Disclosed herein are novel polymer-tethered ligands, metal complexes comprising these ligands, and the use of these complexes as chain transfer catalysts to control the molecular weight of oligomeric and polymeric materials produced in a radical polymerization process. The materials made by the processes disclosed herein have significantly reduced color, making them suitable for a wide range of color-critical end-uses, including automotive coatings.
-
US8030422B2申请人:——公开号:US8030422B2公开(公告)日:2011-10-04
表征谱图
-
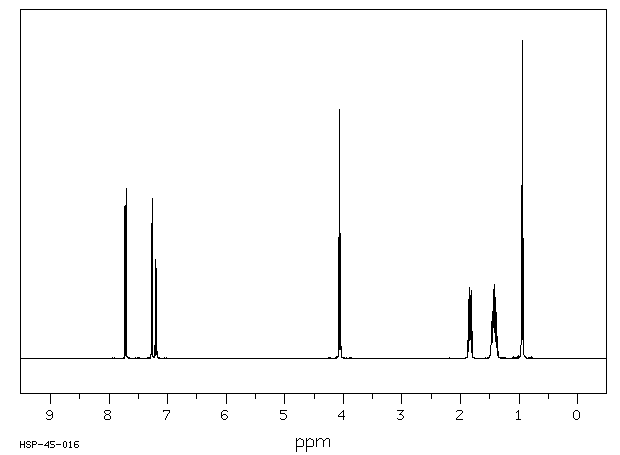
氢谱1HNMR
-
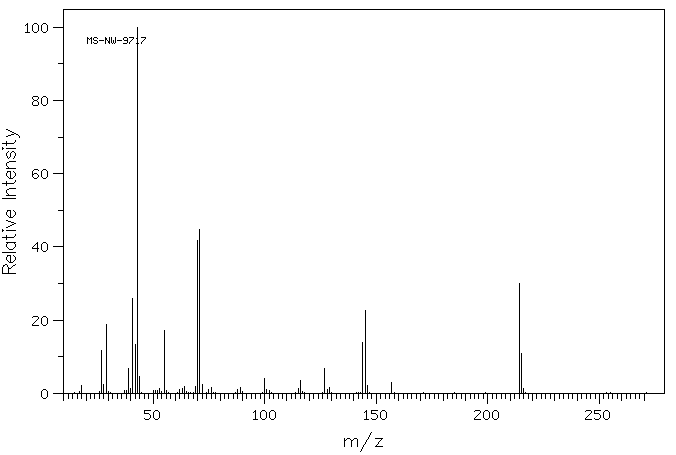
质谱MS
-
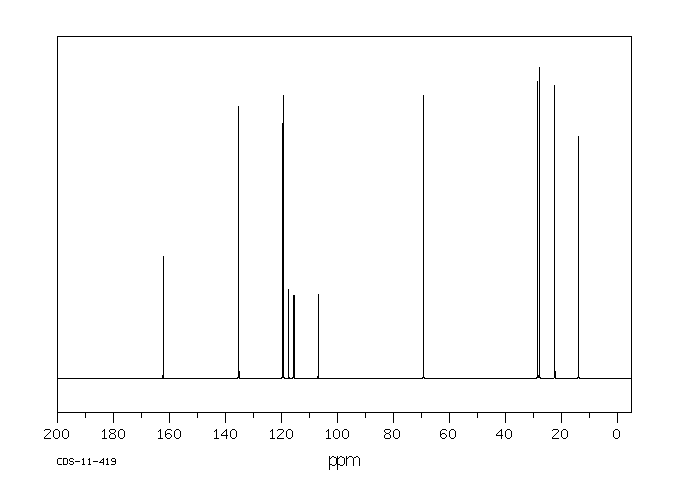
碳谱13CNMR
-
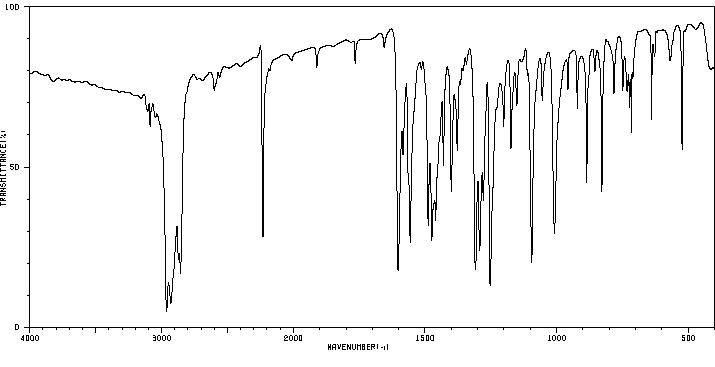
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯