7-iodo-4-methyltropolone | 124616-56-4
中文名称
——
中文别名
——
英文名称
7-iodo-4-methyltropolone
英文别名
2-Hydroxy-3-iodo-6-methylcyclohepta-2,4,6-trien-1-one
CAS
124616-56-4;908104-79-0
化学式
C8H7IO2
mdl
——
分子量
262.047
InChiKey
JVHGHBJWCXDHRM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:11
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-甲基托酚酮 4-methyltropolone 583-80-2 C8H8O2 136.15
反应信息
-
作为反应物:描述:7-iodo-4-methyltropolone 在 platinum(IV) oxide 、 palladium on activated charcoal 、 甲烷 吡啶 、 咪唑 、 氢氧化钾 、 lithium aluminium tetrahydride 、 Amano Lipase PS 、 4 A molecular sieve 、 氢气 、 sodium methylate 、 sodium ethanolate 、 sodium acetate 、 sodium hydride 作用下, 以 甲醇 、 乙醚 、 乙醇 为溶剂, 200.0 ℃ 、101.33 kPa 条件下, 反应 50.04h, 生成 [(2S)-2-[(1S,2S,5S)-2-hydroxy-5-methylcyclohept-3-en-1-yl]propyl] acetate参考文献:名称:A Novel Synthetic Method of the (±)-(3aα,8aα)-Ethyl 8β-Hydroxy-6β-methyl-2-oxooctahydro-2H-cyclohepta[b]furan-3α- carboxylate and Its Chemical Transformation to (±)-(3aα,8aα)-3α,6β-Dimethyl-3,3a,4,5,6,8a-hexahydro-2H- cyclohepta[b]furan-2-one, (+)- and (−)-7β-(2-Acetoxy-1α-methylethyl)-4β-methyl-2-cyclohepten-1β-ol, and (+)- and (−)-7β-(2-Acetoxy-1α-methylethyl)-4β-methyl-2-cyclohepten-1-one. Possible Common Synthetic Intermediates for Pseudoguaianolides, 4,5-Secopseudoguaianolides, Guaianolides, 4,5-Secoguaianolides, and Octalactins摘要:The catalytic hydrogenation of ethyl 8-hydroxy-6-methyl-2-oxo-2H-cyclohepta[b] (6), which was derived regioselectively from 4-methyltropolone (1) in four steps in 62% overall yield, gave (3a alpha,8a alpha)-ethyl 8 beta-hydroxy-6 beta-methyl-2-oxooctahydro-2H-cyclohepta[b]furan-3 alpha-carboxylate (8b) in 45% yield. It is noteworthy that four asymmetric centers newly introduced on the seven-membered ring of 8b were controlled to be syn-oriented by the single operation. The latter was transformed to (3a alpha,8a alpha)-3 alpha,6 beta-dimethyl-3,3a,4,5,6,8a-hexahydro-2H-cyclohepta[b]furan-2-one (17a) in 77% overall yield in five steps, Reduction of 17a with LiAlH4 gave (+/-)-7 beta-(2-hydroxy-1 alpha-methylethyl)-4 beta-methyl-2-cyclohepten-1 beta-ol (22), whose enantioselective acetylation was achieved by vinyl acetate in the presence of Lipase PS to give (+)-7 beta-(2-acetoxy-1 alpha-methylethyl)-4 beta-methyl-2-cyclohepten-1 beta-ol (24) in 48% yield (93% ee) or in 52% yield (77% ee) and (-)-22 in 52% yield (70% ee) or 44% yield (89% ee). Oxidation of (+)-24 with MnO2 gave (-)-7 beta-(2-acetoxy-1 alpha-methyl ethyl)-4 beta-methyl-2-cyclohepten-1-one (-)-(25). Similarly, acetylation of (-)-22 followed by oxidation of resulting (-)-24 gave (+)-25.DOI:10.1021/jo971529q
-
作为产物:描述:4-甲基托酚酮 在 碘 、 potassium carbonate 、 potassium iodide 作用下, 以 水 为溶剂, 反应 20.0h, 以85%的产率得到7-iodo-4-methyltropolone参考文献:名称:A Novel Synthetic Method of the (±)-(3aα,8aα)-Ethyl 8β-Hydroxy-6β-methyl-2-oxooctahydro-2H-cyclohepta[b]furan-3α- carboxylate and Its Chemical Transformation to (±)-(3aα,8aα)-3α,6β-Dimethyl-3,3a,4,5,6,8a-hexahydro-2H- cyclohepta[b]furan-2-one, (+)- and (−)-7β-(2-Acetoxy-1α-methylethyl)-4β-methyl-2-cyclohepten-1β-ol, and (+)- and (−)-7β-(2-Acetoxy-1α-methylethyl)-4β-methyl-2-cyclohepten-1-one. Possible Common Synthetic Intermediates for Pseudoguaianolides, 4,5-Secopseudoguaianolides, Guaianolides, 4,5-Secoguaianolides, and Octalactins摘要:The catalytic hydrogenation of ethyl 8-hydroxy-6-methyl-2-oxo-2H-cyclohepta[b] (6), which was derived regioselectively from 4-methyltropolone (1) in four steps in 62% overall yield, gave (3a alpha,8a alpha)-ethyl 8 beta-hydroxy-6 beta-methyl-2-oxooctahydro-2H-cyclohepta[b]furan-3 alpha-carboxylate (8b) in 45% yield. It is noteworthy that four asymmetric centers newly introduced on the seven-membered ring of 8b were controlled to be syn-oriented by the single operation. The latter was transformed to (3a alpha,8a alpha)-3 alpha,6 beta-dimethyl-3,3a,4,5,6,8a-hexahydro-2H-cyclohepta[b]furan-2-one (17a) in 77% overall yield in five steps, Reduction of 17a with LiAlH4 gave (+/-)-7 beta-(2-hydroxy-1 alpha-methylethyl)-4 beta-methyl-2-cyclohepten-1 beta-ol (22), whose enantioselective acetylation was achieved by vinyl acetate in the presence of Lipase PS to give (+)-7 beta-(2-acetoxy-1 alpha-methylethyl)-4 beta-methyl-2-cyclohepten-1 beta-ol (24) in 48% yield (93% ee) or in 52% yield (77% ee) and (-)-22 in 52% yield (70% ee) or 44% yield (89% ee). Oxidation of (+)-24 with MnO2 gave (-)-7 beta-(2-acetoxy-1 alpha-methyl ethyl)-4 beta-methyl-2-cyclohepten-1-one (-)-(25). Similarly, acetylation of (-)-22 followed by oxidation of resulting (-)-24 gave (+)-25.DOI:10.1021/jo971529q
表征谱图
-
氢谱1HNMR
-
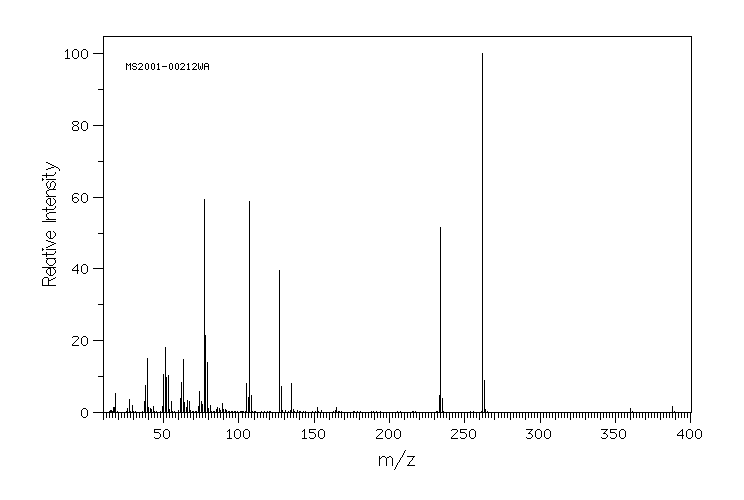
质谱MS
-
碳谱13CNMR
-
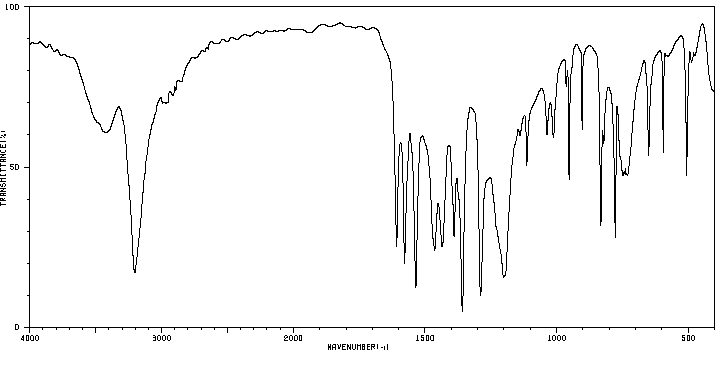
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
脱羰秋水仙碱
红陪酚四甲基醚
红倍酚
秋水仙碱甲硫代磺酸盐
秋水仙碱
硫代秋水仙碱
甲基丙烯酸7-氧代-4-(苯基偶氮)-1,3,5-环庚三烯-1-基酯
甲基6-肼基-7-氧代-1,3,5-环庚三烯-1-羧酸酯
环庚三烯酮
环庚三烯酚酮
氨甲酸,(1-乙基戊基)-,甲基酯(9CI)
桧木醇
异秋水仙胺
尼楚酮
对二硫辛酸
双环[4.4.1]十一碳-1(10),2,4,6,8-五烯-11-酮
双环[4.1.0]庚-1,3,5-三烯-7-酮
去乙酰氨基秋水仙碱
原秋水仙碱
十四烷酸,4-(十八烷氧基)-7-羰基-1,3,5-环庚三烯-1-基酯
乙基[(7S)-1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基]氨基甲酸酯
三甲基秋水仙素酸
三甲基秋水仙素酸
三(2-羟基-2,4,6-环庚三烯-1-酮)-铟
α-(异丙基)-γ,γ-二甲基环己丙醇
beta-斧松素
[(7S)-7-乙酰氨基-1,3-二甲氧基-10-甲硫基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-2-基]2-氯乙酸酯
[(7S)-7-乙酰氨基-1,2-二甲氧基-10-甲硫基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-3-基]2-氯乙酸酯
N-(2-巯基乙基)秋水仙胺
N-脱乙酰基3-去甲基硫代秋水仙碱
N-脱乙酰基,1,2,3,10-脱甲基秋水仙碱
N-甲酰脱乙酰秋水仙碱
N-甲酰基秋水仙胺
N-甲基-秋水仙碱
N-三氟乙酰基-N-甲基-去乙酰基秋水仙碱
N-[(S)-5,6,7,9-四氢-1,2,3,10-四甲氧基-9-氧代苯并[a]庚搭烯-7-基]-2,2,2-三氟乙酰胺
N-[(7S)-4-(羟基甲基)-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-10-(丁基氨基)-5,6,7,9-四氢-1,2,3-三甲氧基-9-氧代苯并[a]庚搭烯-7-基]-乙酰胺
N-[(7S)-1,2,3-三甲氧基-9-氧代-10-(苯基甲硫基)-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-1,2,3-三甲氧基-9-氧代-10-(苯基甲基氨基)-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基]丙酰胺
N-[(7R)-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-(乙氧基乙酰基)去乙酰基硫代秋水仙碱
N-(5,6,7,9-四氢-1,2,3-三甲氧基-10-甲硫基-9-氧代苯并[a]庚搭烯-7-基)氨基甲酸乙酯
N-(4-甲酰基-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(10-二甲基氨基-1,2,3-三甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(1,2,3,9-四甲氧基-10-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基)乙酰胺
9H-三苯并[A,C,E][7]环轮烯-9-酮
8H-环庚三烯并[c]异噻唑-8-酮,3-甲基-