(+/-)-Epinephrine bitartrate | 51-42-3
中文名称
——
中文别名
——
英文名称
(+/-)-Epinephrine bitartrate
英文别名
racemic epinephrine bitartrate;L-(+)-Epinephrine tartrate;epinephrine bitartrate;Adrenaline bitartrate;Adrenalin*Weinsaeure;2,3-Dihydroxybutanedioic acid; 4-[1-hydroxy-2-(methylamino)ethyl]benzene-1,2-diol;2,3-dihydroxybutanedioic acid;4-[1-hydroxy-2-(methylamino)ethyl]benzene-1,2-diol
CAS
51-42-3;636-89-5;1234-55-5;2964-12-7;14479-84-6;19947-47-8;21282-30-4;24351-82-4;40004-76-0;55750-10-2;69207-54-1;104655-01-8
化学式
C4H6O6*C9H13NO3
mdl
——
分子量
333.295
InChiKey
YLXIPWWIOISBDD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):-1.77
-
重原子数:23
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.38
-
拓扑面积:188
-
氢给体数:8
-
氢受体数:10
安全信息
-
危险等级:6.1(b)
-
危险品标志:T+
-
安全说明:S26,S28,S36/37,S45
-
危险类别码:R36/37/38,R28
-
WGK Germany:3
-
海关编码:29379000
-
RTECS号:DO3500000
-
包装等级:III
-
危险类别:6.1(b)
-
危险品运输编号:UN 2811 6.1/PG 1
-
储存条件:密封避光保存。
SDS
Section 1. Chemical Product and Company Identification
Epinephrine bitartrate Catalog
EP135
Common Name/
Number(s).
Trade Name
CAS# 51-42-3
Manufacturer
RTECS Not available.
SPECTRUM CHEMICAL MFG. CORP.
TSCA TSCA 8(b) inventory:
Epinephrine bitartrate
Commercial Name(s) Not available.
CI# Not available.
Synonym L-Adrenaline
IN CASE OF EMERGENCY
Not available.
Chemical Name
Chemical Family Not available. CALL (310) 516-8000
C9H13NO3.C4H6O6
Chemical Formula
SPECTRUM CHEMICAL MFG. CORP.
Section 2.Composition and Information on Ingredients
Exposure Limits
TWA (mg/m3) STEL (mg/m3) CEIL (mg/m3)
Name CAS # % by Weight
1) Epinephrine bitartrate 51-42-3 100
Toxicological Data Epinephrine bitartrate:
on Ingredients ORAL (LD50): Acute: 4 mg/kg [Mouse].
Section 3. Hazards Identification
Potential Acute Health Effects Very hazardous in case of ingestion, of inhalation. Hazardous in case of skin contact (irritant, permeator), of eye
contact (irritant). Severe over-exposure can result in death.
Potential Chronic Health Very hazardous in case of ingestion, of inhalation.
Hazardous in case of skin contact (irritant, permeator), of eye contact (irritant).
Effects
CARCINOGENIC EFFECTS: Not available.
MUTAGENIC EFFECTS: Not available.
TERATOGENIC EFFECTS: Not available.
DEVELOPMENTAL TOXICITY: Not available.
The substance is toxic to lungs, the nervous system, mucous membranes.
Repeated or prolonged exposure to the substance can produce target organs damage. Repeated exposure to an
highly toxic material may produce general deterioration of health by an accumulation in one or many human
organs.
Epinephrine bitartrate
Section 4. First Aid Measures
Eye Contact Check for and remove any contact lenses. Immediately flush eyes with running water for at least 15 minutes,
keeping eyelids open. Cold water may be used. Do not use an eye ointment. Seek medical attention.
Skin Contact After contact with skin, wash immediately with plenty of water. Gently and thoroughly wash the contaminated skin
with running water and non-abrasive soap. Be particularly careful to clean folds, crevices, creases and groin.
Cold water may be used. Cover the irritated skin with an emollient. If irritation persists, seek medical attention.
Wash contaminated clothing before reusing.
Serious Skin Contact Wash with a disinfectant soap and cover the contaminated skin with an anti-bacterial cream. Seek immediate
medical attention.
Inhalation Allow the victim to rest in a well ventilated area. Seek immediate medical attention.
Serious Inhalation Evacuate the victim to a safe area as soon as possible. Loosen tight clothing such as a collar, tie, belt or
waistband. If breathing is difficult, administer oxygen. If the victim is not breathing, perform mouth-to-mouth
resuscitation. Seek medical attention.
Ingestion Do not induce vomiting. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible
indication that the toxic material was ingested; the absence of such signs, however, is not conclusive. Loosen
tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth
resuscitation. Seek immediate medical attention.
Serious Ingestion
Not available.
Section 5. Fire and Explosion Data
Flammability of the Product May be combustible at high temperature.
Auto-Ignition Temperature Not available.
Flash Points Not available.
Flammable Limits Not available.
Products of Combustion These products are carbon oxides (CO, CO2), nitrogen oxides (NO, NO2...).
Fire Hazards in Presence of Not available.
Various Substances
Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available.
Risks of explosion of the product in presence of static discharge: Not available.
of Various Substances
SMALL FIRE: Use DRY chemical powder.
Fire Fighting Media
and Instructions LARGE FIRE: Use water spray, fog or foam. Do not use water jet.
Not available.
Special Remarks on
Fire Hazards
Special Remarks on Explosion Not available.
Hazards
Section 6. Accidental Release Measures
Small Spill Use appropriate tools to put the spilled solid in a convenient waste disposal container.
Large Spill Use a shovel to put the material into a convenient waste disposal container.
Epinephrine bitartrate
Section 7. Handling and Storage
Precautions Keep locked up Keep away from heat. Keep away from sources of ignition. Empty containers pose a fire risk,
evaporate the residue under a fume hood. Ground all equipment containing material. Do not ingest. Do not
breathe dust. Wear suitable protective clothing In case of insufficient ventilation, wear suitable respiratory
equipment If ingested, seek medical advice immediately and show the container or the label. Avoid contact with
skin and eyes
Storage Keep container dry. Keep in a cool place. Ground all equipment containing material. Keep container tightly
closed. Keep in a cool, well-ventilated place. Highly toxic or infectious materials should be stored in a separate
locked safety storage cabinet or room.
Section 8. Exposure Controls/Personal Protection
Engineering Controls Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below
recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to
airborne contaminants below the exposure limit.
Personal Protection Splash goggles. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent.
Gloves.
Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used
a Large Spill to avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist
BEFORE handling this product.
Exposure Limits Not available.
Section 9. Physical and Chemical Properties
Physical state and appearance Solid. (Crystalline solid.) Odor Not available.
Taste Not available.
333.29 g/mole
Molecular Weight
Color Not available.
pH (1% soln/water) Not available.
Not available.
Boiling Point
Melting Point Decomposes. (147°C or 296.6°F)
Not available.
Critical Temperature
Specific Gravity Not available.
Vapor Pressure Not applicable.
Vapor Density Not available.
Volatility Not available.
Odor Threshold Not available.
Water/Oil Dist. Coeff. Not available.
Not available.
Ionicity (in Water)
Dispersion Properties See solubility in water.
Solubility Soluble in cold water.
Epinephrine bitartrate
Section 10. Stability and Reactivity Data
The product is stable.
Stability
Instability Temperature Not available.
Not available.
Conditions of Instability
Not available.
Incompatibility with various
substances
Corrosivity Non-corrosive in presence of glass.
Special Remarks on Not available.
Reactivity
Special Remarks on Not available.
Corrosivity
Polymerization No.
Section 11. Toxicological Information
Routes of Entry Dermal contact. Eye contact. Inhalation. Ingestion.
Toxicity to Animals Acute oral toxicity (LD50): 4 mg/kg [Mouse].
Chronic Effects on Humans The substance is toxic to lungs, the nervous system, mucous membranes.
Other Toxic Effects on Very hazardous in case of ingestion, of inhalation.
Humans Hazardous in case of skin contact (irritant, permeator).
Special Remarks on Not available.
Toxicity to Animals
Special Remarks on Not available.
Chronic Effects on Humans
Special Remarks on other Not available.
Toxic Effects on Humans
Section 12. Ecological Information
Ecotoxicity Not available.
BOD5 and COD Not available.
Products of Biodegradation Possibly hazardous short term degradation products are not likely. However, long term degradation products may
arise.
The products of degradation are more toxic.
Toxicity of the Products
of Biodegradation
Special Remarks on the Not available.
Products of Biodegradation
Epinephrine bitartrate
Section 13. Disposal Considerations
Waste Disposal
Section 14. Transport Information
DOT Classification CLASS 6.1: Poisonous material.
: Toxic solid, organic, n.o.s. (Epinephrine bitartrate) : UN2811 PG: III
Identification
Not available.
Special Provisions for
Transport
DOT (Pictograms)
HARMFUL
STOW AWAY
FROM
FOODSTUFFS
6
Section 15. Other Regulatory Information and Pictograms
TSCA 8(b) inventory: Epinephrine bitartrate
Federal and State
Regulations
California
Proposition 65
Warnings
Other Regulations OSHA: Hazardous by definition of Hazard Communication Standard (29 CFR 1910.1200).
CLASS D-1A: Material causing immediate and serious toxic effects (VERY TOXIC).
Other Classifications WHMIS (Canada)
CLASS D-2A: Material causing other toxic effects (VERY TOXIC).
DSCL (EEC) R36/38- Irritating to eyes and skin.
Health Hazard
HMIS (U.S.A.) 4 National Fire Protection
1 Flammability
1 Association (U.S.A.)
Fire Hazard
4 0 Reactivity
Health
Reactivity
0
Specific hazard
Personal Protection
E
WHMIS (Canada)
(Pictograms)
DSCL (Europe)
(Pictograms)
TDG (Canada)
(Pictograms)
6
Epinephrine bitartrate
ADR (Europe)
(Pictograms)
Protective Equipment
Gloves.
Lab coat.
Dust respirator. Be sure to use an
approved/certified respirator or
equivalent. Wear appropriate respirator
when ventilation is inadequate.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
反应信息
-
作为反应物:描述:(+/-)-Epinephrine bitartrate 在 N-乙酰基-L-半胱氨酸 作用下, 生成 Leukoadrenochrom 、 alkaline earth salt of/the/ methylsulfuric acid参考文献:名称:Raether; Lebus; Klopsch, Pharmazie, 1991, vol. 46, # 6, p. 426 - 431摘要:DOI:
文献信息
-
Multi-functional ionic liquid compositions for overcoming polymorphism and imparting improved properties for active pharmaceutical, biological, nutritional, and energetic ingredients申请人:Rogers D. Robin公开号:US20070093462A1公开(公告)日:2007-04-26Disclosed are ionic liquids and methods of preparing ionic liquid compositions of active pharmaceutical, biological, nutritional, and energetic ingredients. Also disclosed are methods of using the compositions described herein to overcome polymorphism, overcome solubility and delivery problems, to control release rates, add functionality, enhance efficacy (synergy), and improve ease of use and manufacture.
-
Dendrimers as molecular translocators申请人:Goodman Murray公开号:US20060216265A1公开(公告)日:2006-09-28Transport molecules include a dendrimer and a biologically active molecule. The dendrimer of such transport molecules includes at least one guanidine group, at least one protonated guanidine group, at least one protected guanidine group, at least one amidine group, at least one protonated amidine group, at least one protected amidine group, at least one ureido group, at least one protonated ureido group, at least one protected ureido group, at least one thioureido group, at least one protonated thioureido group, or at least one protected thioureido group. The biologically active molecule is bonded to the dendrimer. A method of increasing the bioavailability of a drug includes bonding the drug to a dendrimer of the invention.
-
[EN] PROCESS FOR THE PREPARATION OF OPTICALLY ENRICHED ADRENALINE<br/>[FR] PROCÉDÉ DE PRÉPARATION D'ADRÉNALINE ENRICHIE OPTIQUEMENT申请人:ROUVER INVEST S À R L公开号:WO2016038422A1公开(公告)日:2016-03-17A process for the production of (-)-adrenaline and (-)-adrenaline-L-tartrate is provided. The process provides for a new, efficient and commercially feasible process for the optical resolution of racemic adrenaline. In one aspect, a one pot process for the synthesis of (-)-adrenaline is provided. The process provides a simple and less expensive 5 process that can be used to prepare commercial scale batches of (-)-adrenaline and (-)-adrenaline-L-tartrate of API quality. The process avoids the use of expensive and unpredictable chiral catalysts.
-
CRYSTALLINE EPINEPHRINE MALONATE SALT申请人:Biothea Pharma, Inc.公开号:US20200031758A1公开(公告)日:2020-01-30Described herein are epinephrine salts, specifically the epinephrine malonate salt; the epinephrine malonate salt in crystalline form; a pharmaceutical composition comprising epinephrine malonate; a sublingual or buccal pharmaceutical composition comprising epinephrine malonate in crystalline form; and a method for treating a patient comprising administering a pharmaceutical composition of epinephrine malonate in crystalline form.
-
[EN] HYPOGLYCEMIC DIHYDROPYRIDONES<br/>[FR] DIHYDROPYRIDONES HYPOGLYCÉMIQUES申请人:UNIV COLUMBIA公开号:WO2010016939A1公开(公告)日:2010-02-11The present invention provides, inter alia, a compound of formula I: wherein the substituent designations are indicated in the Specification. The present invention also provides pharmaceutical compositions comprising a compound of formula I, and methods of treatment or prevention of diabetes or hyperglycemia in a patient, and of normalizing blood glucose levels in a subject, by administering an effective amount of a compound of formula I.
表征谱图
-
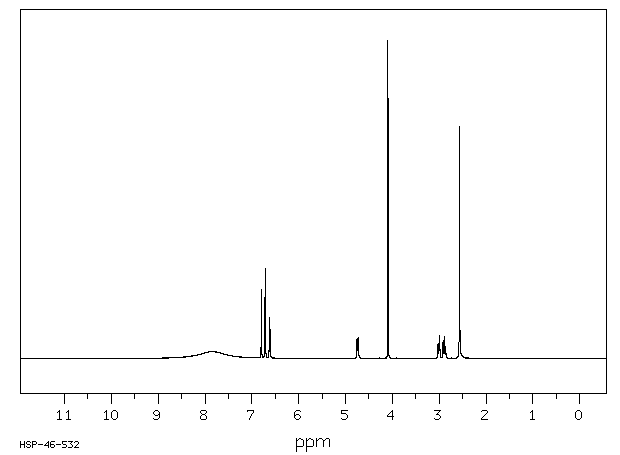
氢谱1HNMR
-
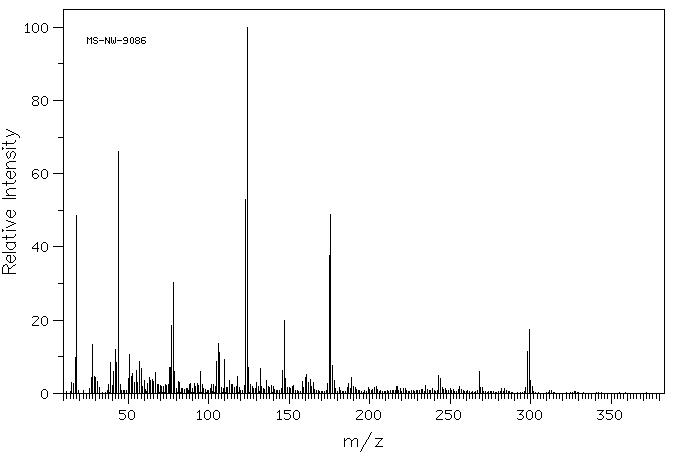
质谱MS
-
碳谱13CNMR
-
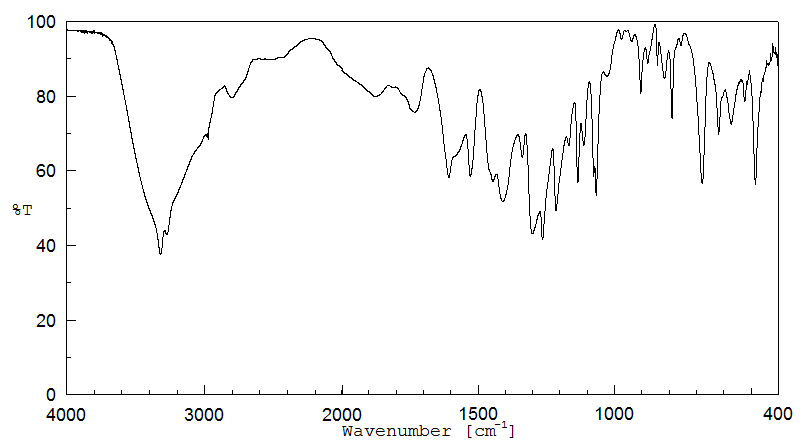
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚