1,4-dihydro-2,3-benzoxathiin 3-oxide | 51439-46-4
中文名称
——
中文别名
——
英文名称
1,4-dihydro-2,3-benzoxathiin 3-oxide
英文别名
1,4-dihydrobenzo[d][1,2]oxathiine 3-oxide;4,5-benzo-3,6-dihydro-1,2-oxathiin-2-oxide;1,4-Dihydro-2,3-benzoxathiin-3-oxide;1,4-dihydro-2,3λ4-benzoxathiine 3-oxide
CAS
51439-46-4
化学式
C8H8O2S
mdl
——
分子量
168.216
InChiKey
NIXVNZNYHSLXMV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:112-114 °C (sublm)
-
沸点:328.3±52.0 °C(Predicted)
-
密度:1.39±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:45.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2934999090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Thermodynamically stable [4 + 2] cycloadducts of lanthanum-encapsulated endohedral metallofullerenes摘要:通过从4,5-苯并-3,6-二氢-1,2-噁硫环己烯-2-氧化物及其衍生物生成的现场产生的o-喹诺二甲烷的[4 + 2]环加成反应,与镧金属封装富勒烯La2@C80或La@C82反应,形成了新颖的内囊金属富勒烯衍生物(3a、b、4a、b和5b)。通过MALDI-TOF质谱、光吸收和NMR光谱等光谱方法阐明了所得化合物的分子结构。分别对La2@C80(3a、b和4a、b)和La@C82(5b)的[4 + 2]加合物进行了EPR光谱确认,保留了顺磁和顺磁性质。在不同温度下对4a的动态NMR测量显示了加成物的船对船倒转。此外,与报道的五甲基环戊二烯和La@C82(6)的[4 + 2]环加成物相比,5b表现出显著的热稳定性。这些发现证明了苯并硫醚的实用性,可提供热力学稳定的内囊金属富勒烯衍生物,用于材料科学中的应用。DOI:10.3762/bjoc.10.65
-
作为产物:描述:1-hydroxy-1,3-dihydrobenzo
thiophene 2,2-dioxide 在 sodium tetrahydroborate 作用下, 以 甲醇 为溶剂, 反应 0.5h, 以70%的产率得到1,4-dihydro-2,3-benzoxathiin 3-oxide参考文献:名称:Durst, Tony; Charlton, James L.; Mount, David B., Canadian Journal of Chemistry, 1986, vol. 64, p. 246 - 249摘要:DOI: -
作为试剂:描述:甲基苯醌 在 1,4-dihydro-2,3-benzoxathiin 3-oxide 、 四氯化钛 作用下, 以 二氯甲烷 、 苯 为溶剂, 反应 28.0h, 生成 N,N'-dicyano-2-methyl-1,4-anthraquinonediimine参考文献:名称:Syntheses, electrochemistry and molecular modeling of N,N′-dicyanoquinonediimine (DCNQI) derivatives of substituted 1,4-anthracenediones: precursors for organic metals.摘要:The title compounds (10) have been obtained from the corresponding 1,4-anthracenedione derivatives (3), prepared by different synthetic routes, by reaction with bis(trimethylsilyl)carbodiimide (BTC). The cyclic voltammetry of these compounds (3 and 10) reveals the presence of two reduction waves to the corresponding anion-radical and dianion. The acceptor ability of compounds 10 can be modulated by the presence of substituents on the DCNQI ring. The syn/anti isomers have been studied by molecular mechanics and the results are in good agreement with the H-1-NMR high resolution spectral data.DOI:10.1016/s0040-4020(01)80405-1
文献信息
-
Laticyclic Conjugated Polyenes. Study on Diels−Alder Cycloadditions of a Facially Dissymmetric Maleic Anhydride作者:Teh-Chang Chou、Tzong-Shing Jiang、Jenn-Tsang Hwang、Kuan-Jiuh Lin、Cheng-Tung LinDOI:10.1021/jo990256r日期:1999.6.1obtained from the Diels-Alder cycloaddition of the bicyclic ring-fused cyclohexadiene 1 and acetylenedicarboxylate. Maleic anhydride 2 readily underwent the Diels-Alder cycloadditions with anthracene, 1,3-diphenylisobenzofuran, cyclopentadiene, 1,3-cyclohexadiene, 6,6-dimethylfulvene, and o-quinodimethane. All the cycloadditions occurred exclusively on the pi-face syn to the etheno bridge of 2, thereby
-
Synthesis and Characterization of a Novel<i>Diels</i>-<i>Alder</i>Adduct of Codeine作者:Christopher W. Cunningham、Kellie Hom、Chayan Acharya、Angela Wilks、Alexander D. MacKerell、Andrew CoopDOI:10.1002/hlca.200900234日期:2010.2The Diels–Alder reaction was applied to 4,5‐epoxymorphinan opioids to generate a novel aromatic cycloadduct at C(7)C(8): Thermolytic cleavage of sultine 8 produced the reactive diene o‐quinodimethane 7 which condensed favorably with codeine (11), but not with codeinone (9) or 14‐hydroxycodeinone (10), producing the desired tetrahydronaphtho adduct 12 with (7R,8R) geometry (Scheme). The configuration
-
Benzosulfones as photochemically activated sulfur dioxide (SO<sub>2</sub>) donors作者:Satish R. Malwal、Harinath ChakrapaniDOI:10.1039/c4ob02466d日期:——Sulfur dioxide (SO2) is a gaseous environmental pollutant which is routinely used in industry as a preservative and antimicrobial. Recent data suggests that SO2 may have value as a therapeutic agent. However, due to its gaseous nature, localizing SO2 generation is challenging. Herein, various 1,3-dihydrobenzo[c]thiophene 2,2-dioxides (benzosulfones) were prepared as candidates for photochemically activated
-
o-quinodimethanes from 3,6-dihydrobenzo[b]-1,2-oxathiin-2-oxides作者:James L. Charlton、Tony DurstDOI:10.1016/s0040-4039(01)81585-9日期:——o-tolual-dehyde via the photochemically produced 1-hydroxy-1,3-dihydrobenzo[b]thiophene-2,2-dioxide is described. Analogous syntheses of 3 and 6 substituted derivatives of the benzosultines 5 have also been carried out. The feasibility of using these benzosultines as o-quinodimethane precursors has been tested by thermalizing the phenyl sultine 5c in refluxing cyclohexane in the presence of dimethyl fumarate, dimethyl
-
Divergent Approach to the Bisanthraquinone Natural Products: Total Synthesis of (<i>S</i>)-Bisoranjidiol and Derivatives from Binaphtho-<i>para</i>-quinones作者:Erin E. Podlesny、Marisa C. KozlowskiDOI:10.1021/jo302364h日期:2013.1.18development of the first asymmetric synthesis of a chiral anthraquinone dimer is outlined, resulting in the first total synthesis of (S)-bisoranjidiol. Rather than a biomimetic dimerization retrosynthetic disconnection, the anthracenyl ring systems are generated after formation of the axially chiral binaphthalene framework. This synthetic strategy has enabled the synthesis of several analogues. Key
表征谱图
-
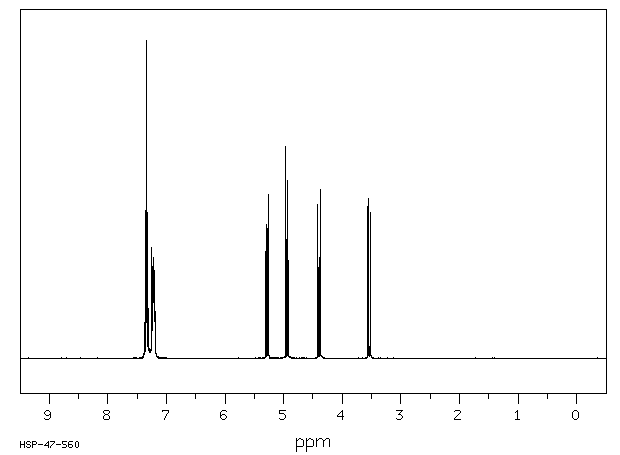
氢谱1HNMR
-
质谱MS
-
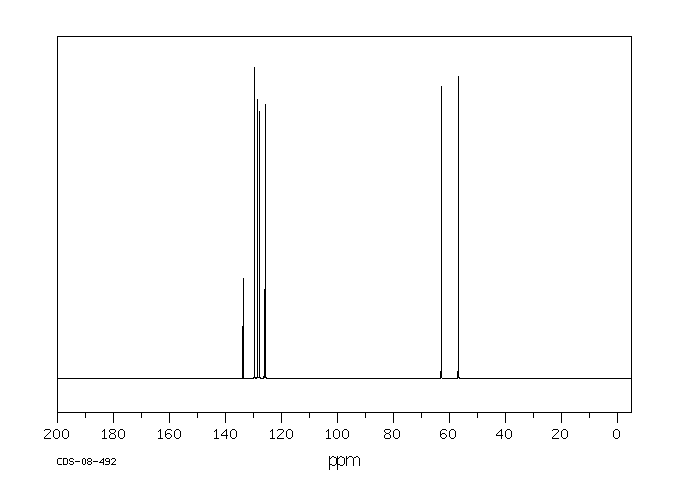
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-(+)-5,5'',6,6'',7,7'',8,8''-八氢-3,3''-二叔丁基-1,1''-二-2-萘酚,双钾盐
(S)-盐酸沙丁胺醇
(S)-溴烯醇内酯
(S)-7,7-双[(4S)-(苯基)恶唑-2-基)]-2,2,3,3-四氢-1,1-螺双茚满
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2-N-Fmoc-氨基甲基吡咯烷盐酸盐
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-7,7-双[(4S)-(苯基)恶唑-2-基)]-2,2,3,3-四氢-1,1-螺双茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(6,6)-苯基-C61己酸甲酯
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,5R)-3,3a,8,8a-四氢茚并[1,2-d]-1,2,3-氧杂噻唑-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aS,8aR)-2-(吡啶-2-基)-8,8a-二氢-3aH-茚并[1,2-d]恶唑
(3aS,3''aS,8aR,8''aR)-2,2''-环戊二烯双[3a,8a-二氢-8H-茚并[1,2-d]恶唑]
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(3-三苯基甲氨基甲基)吡啶
(3-[(E)-1-氰基-2-乙氧基-2-hydroxyethenyl]-1-氧代-1H-茚-2-甲酰胺)
(2′′-甲基氨基-1,1′′-联苯-2-基)甲烷磺酰基铝(II)二聚体
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,4S)-Fmoc-4-三氟甲基吡咯烷-2-羧酸
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环