2,3-diphenylcyclopropen-1-thione | 2570-01-6
中文名称
——
中文别名
——
英文名称
2,3-diphenylcyclopropen-1-thione
英文别名
2,3-diphenylcyclo-2-ene-1-thione;diphenylcyclopropenthione;2,3-diphenylcyclopropenethione;2,3-diphenylcycloprop-2-ene-1-thione;diphenyl-cyclopropenethione;2-Cyclopropen-1-thione, 2,3-diphenyl-
CAS
2570-01-6
化学式
C15H10S
mdl
——
分子量
222.31
InChiKey
QDWQPYYTOPIUBB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:127-128 °C(Solv: cyclohexane (110-82-7))
-
沸点:373.6±52.0 °C(Predicted)
-
密度:1.19 g/cm3
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:16
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:32.1
-
氢给体数:0
-
氢受体数:1
SDS
上下游信息
反应信息
-
作为反应物:描述:2,3-diphenylcyclopropen-1-thione 在 triphenyl phosphite ozonide 作用下, 以 二氯甲烷 为溶剂, 反应 1.0h, 以75%的产率得到二苯基环丙烯酮参考文献:名称:Strain assisted .alpha.-cleavage reactions of thio ketones: diphenylcyclopropenethione摘要:DOI:10.1021/jo00139a036
-
作为产物:参考文献:名称:Acylthiocyclopropenium Ions摘要:在醋酸酐中用硫代羧酸和高氯酸的混合物处理环丙烯酮,可以得到良产率的酰基硫环丙烯阳离子高氯酸盐。在类似反应条件下,双(酰基硫)环丙烯也能得到该盐。该盐在氮气气氛下低温下稳定,但易水解生成相应的环丙烯硫酮。DOI:10.1246/bcsj.55.1973
文献信息
-
A Cyclopropenethione-Phosphine Ligation for Rapid Biomolecule Labeling作者:R. David Row、Jennifer A. PrescherDOI:10.1021/acs.orglett.8b02296日期:2018.9.21Cyclopropenethiones are reported as new bioorthogonal reagents. These motifs react readily with substituted phosphines to provide thiocarbonyl adducts. In some cases, the ligations are >300-fold faster than analogous reactions with bioorthogonal cyclopropenones. Dialkyl cyclopropenethiones are also stable in aqueous buffers and can be used for biomolecule labeling in vitro and in cell lysate. The rapid
-
Stereospecific Reactions of Donor-Acceptor Cyclopropanes with Thioketones: Access to Highly Substituted Tetrahydrothiophenes作者:André U. Augustin、Maximilian Sensse、Peter G. Jones、Daniel B. WerzDOI:10.1002/anie.201708346日期:2017.11.6Lewis‐acid‐catalyzed reactions of 2‐substituted cyclopropane 1,1‐dicarboxylates with thioketones are described. Highly substituted tetrahydrothiophenes with two adjacent quaternary carbon atoms were obtained in a stereospecific manner under mild conditions and in high yield when using AlCl3 as Lewis acid. Moreover, an intramolecular approach was successfully implemented to gain access to sulfur‐bridged
-
A NOVEL CYCLISATION REACTION OF ALKYLTHIODIPHENYLCYCLOPROPENIUM IONS WITH 1,3-DIKETONES TO GIVE CYCLOPENTADIENOLS作者:Hiroshi Yoshida、Mikito Nakajima、Tsuyoshi Ogata、Kiyoshi Matsumoto、R. Morrin Acheson、John D. WallisDOI:10.1246/cl.1983.155日期:1983.2.5Alkylthiodiphenylcyclopropenium ions reacted with 2,4-pentanedione or ethyl acetoacetate to give cyclopentadienol derivatives by ring expansion, while triphenylcyclopropenium perchlorate yielded substituted cyclopropenes.
-
A Novel Cyclization Reaction of Alkylthiodiphenylcyclopropenium Ions with Acyclic 1,3-Diketones to Give Cyclopentadienols作者:Hiroshi Yoshida、Mikito Nakajima、Tsuyoshi Ogata、Kiyoshi Matsumoto、R. Morrin Acheson、John D. WallisDOI:10.1246/bcsj.56.3015日期:1983.102,4-pentanedione (3a) and ethyl acetoacetate (3b) yielded the cyclopentadienol derivatives (4) by ring expansion. One of the products 4a was shown to be 4-acetyl-5-hydroxy-5-methyl-1-methylthio-2,3-diphenyl-1,3-cyclopentadiene by X-ray crystallography. Chemical transformation of the products yielded some cyclopentenones. Triphenylcyclopropenium perchlorate reacted with 3a and 3b affording the ketocyclopropene
-
Reaction of Diphenylcyclopropenethione with Pyridinium Imines作者:J. W. Lown、K. MatsumotoDOI:10.1139/v72-090日期:1972.2.15Reaction of diphenylcyclopropenethione with a variety of N-substituted pyridinium imines in refluxing benzene gives 2,4,5-trisubstituted-6H-1,3-oxazin-6-thiones in good to excellent yields. The structure of 2,4,5-triphenyl-6H-1,3-oxazin-6-thione prepared in this manner was proven by oxidation and by hydrolysis to the known 2,4,5-triphenyl-6H-1,3-oxazin-6-one. In the preparation of 4,5-diphenyl-2-ethoxy-6H-1
表征谱图
-
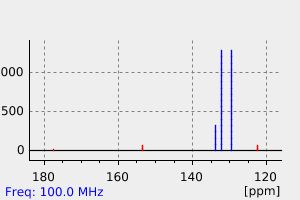
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫