(E)-3-thiophen-2-yl-1-p-tolylpropenone | 6028-89-3
中文名称
——
中文别名
——
英文名称
(E)-3-thiophen-2-yl-1-p-tolylpropenone
英文别名
1-(4-methylphenyl)-3-(2-thienyl)prop-2-en-1-one;3-(Furan-2′′-yl)-1-p-tolylpropenone;(E)-3-(thiophen-2-yl)-1-(p-tolyl)prop-2-en-1-one;1-(2-Thienyl)-3-(4-methylphenyl)-propen-3-on;1-(p-Methylphenyl)-3-(2-thienyl)-propenon;3t-[2]thienyl-1-p-tolyl-propenone;3t-[2]Thienyl-1-p-tolyl-propenon;(E)-3-(2-thienyl)-1-(p-tolyl)-2-propen-1-one;3-thiophen-2-yl-1-p-tolyl-propenone;4'-Methyl-3-(2-thienyl)acrylophenone;(E)-1-(4-methylphenyl)-3-thiophen-2-ylprop-2-en-1-one
CAS
6028-89-3
化学式
C14H12OS
mdl
MFCD00022532
分子量
228.315
InChiKey
OUDDTWPBSXBJOY-CMDGGOBGSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:75 °C
-
沸点:381.5±42.0 °C(Predicted)
-
密度:1.167±0.06 g/cm3(Predicted)
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.071
-
拓扑面积:45.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
WGK Germany:1
-
RTECS号:UP8225000
-
海关编码:29322980
-
危险类别码:R22
-
储存条件:请将药品存放在避光、阴凉干燥处,并密封保存。
SDS
反应信息
-
作为反应物:描述:(E)-3-thiophen-2-yl-1-p-tolylpropenone 在 N-甲基吗啉 、 氢氧化钾 、 2,4,6-三甲基苯磺酰羟胺 作用下, 以 乙腈 为溶剂, 反应 4.0h, 以54%的产率得到参考文献:名称:胺促进的查尔酮的叠氮化。摘要:DOI:10.1002/anie.200602586
-
作为产物:参考文献:名称:Evaluation of Silica-H2SO4 as an Efficient Heterogeneous Catalyst for the Synthesis of Chalcones摘要:我们报道了一种高效的二氧化硅-H2SO4介导的合成多种查尔酮的方法,该方法与碱催化的无溶剂条件以及酸或碱催化的回流条件相比,能够以非常好的产率获得目标化合物。DOI:10.3390/molecules180810081
文献信息
-
Studies on the Nuclear Magnetic Resonance Spectra of (E)-1-Aryl-3-(2- and 3-thienyl)-2-propenones and Unique Observation of<sup>4</sup>J and<sup>5</sup>J Coupling in Their<sup>1</sup>H-<sup>1</sup>H COSY作者:In-Sook HanLee、Hyun-Ju Jeon、Chang-Kiu LeeDOI:10.5012/bkcs.2011.32.2.687日期:2011.2.20
$^1H$ and$^13}C$ NMR spectra of series of (E)-1-aryl-(2- and 3-thienyl)-2-propenones, that are aldol condensation products between 2- and 3-thiophenecarbaldehydes and m- and p-substituted acetophenones, were examined to make complete assignments of the chemical shifts. Long range couplings,$^4J$ and$^5J$ , are observed in the$^1H-^1H$ COSY of both 2- and 3-thienyl compounds, which makes the elucidation of the conformation in solution possible. In contrast, the 2-furyl analogue shows the long range coupling phenomena, but the 3-furyl and phenyl analogues do not show similar phenomena. -
Novel One-Step Synthesis of Thiazolo[3,2-<i>b</i>]1,2,4-triazoles作者:Alan R. Katritzky、Alfredo Pastor、Michael Voronkov、Peter J. SteelDOI:10.1021/ol990370w日期:2000.2.1[reaction: see text] Reactions of chalcones 3a-f with bis(1H-1,2,4-triazolyl) sulfoxide 4 formed the thiazolo[3,2-b]1,2,4-triazoles 5a-f, which resemble closely some previously prepared COX-2 inhibitors. The structure of 5a was confirmed by X-ray analysis.[反应:参见文本]查耳酮3a-f与双(1H-1,2,4-三唑基)亚砜4的反应形成噻唑并[3,2-b] 1,2,4-三唑5a-f,类似于紧密配合一些先前制备的COX-2抑制剂。通过X射线分析确认了5a的结构。
-
<i>N</i>-Heterocyclic Carbene-Catalyzed Reaction of Chalcones and Enals via Homoenolate: an Efficient Synthesis of 1,3,4-Trisubstituted Cyclopentenes作者:Vijay Nair、Sreekumar Vellalath、Manojkumar Poonoth、Eringathodi SureshDOI:10.1021/ja0625677日期:2006.7.1Nucleophilic heterocyclic carbene-catalyzed cyclopentannulation of enals and chalcones via homoenolate has been observed for the first time. Serendipitously, the reaction lead to a very efficient synthesis of 3,4-trans-disubstituted-1-aryl cyclopentenes instead of the expected cyclopentanones. The strategy works well with thienylidene tetralone also, leading to the tricyclic cyclopentene derivative
-
Transition-Metal-Free Synthesis of Homo- and Hetero-1,2,4-Triaryl Benzenes by an Unexpected Base-Promoted Dearylative Pathway作者:Mohammad Rehan、Sanjay Maity、Lalit Kumar Morya、Kaushik Pal、Prasanta GhoraiDOI:10.1002/anie.201511424日期:2016.6.27γ‐di‐aryl propanones. The salient feature of this strategy involves the sequential hydride transfer, regiospecific condensation, regiospecific dearylation, and aromatization under metal‐free reaction conditions. The synthesis of unsymmetrically substituted triphenylenes by oxidative coupling of the synthesized 1,2,4‐triaryl benzenes has also been demonstrated.
-
Synthesis and Anti-Bacterial Activity of Some Heterocyclic Chalcone Derivatives Bearing Thiofuran, Furan, and Quinoline Moieties作者:Chang-Ji Zheng、Sheng-Ming Jiang、Zhen-Hua Chen、Bai-Jun Ye、Hu-Ri PiaoDOI:10.1002/ardp.201100005日期:2011.1036 Novel heterocyclic chalcone derivatives were synthesized and tested for their anti‐bacterial activity. Some compounds presented good anti‐microbial activities against Gram‐positive bacteria (including the multidrug‐resistant clinical isolates). This class of compounds presented high potency against Streptococcus mutans, among which the derivatives F2 with an MIC of 2 µg/mL was as active as the standard
表征谱图
-
氢谱1HNMR
-
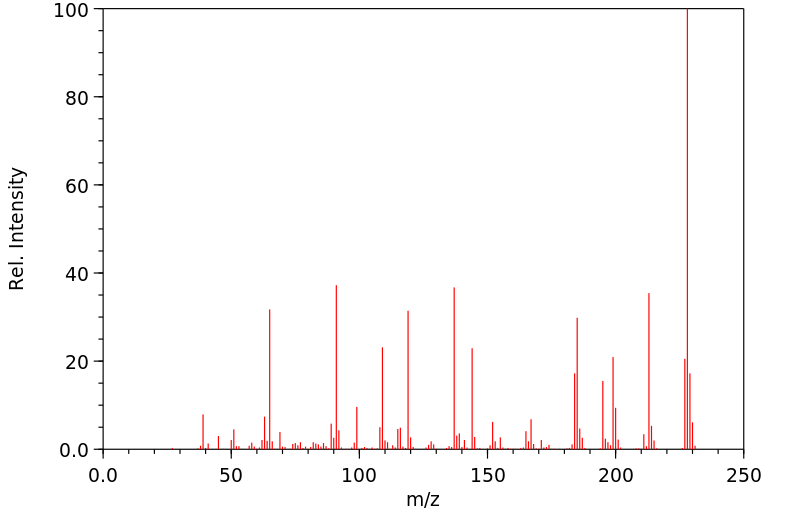
质谱MS
-
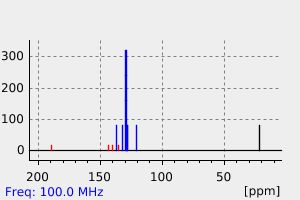
碳谱13CNMR
-
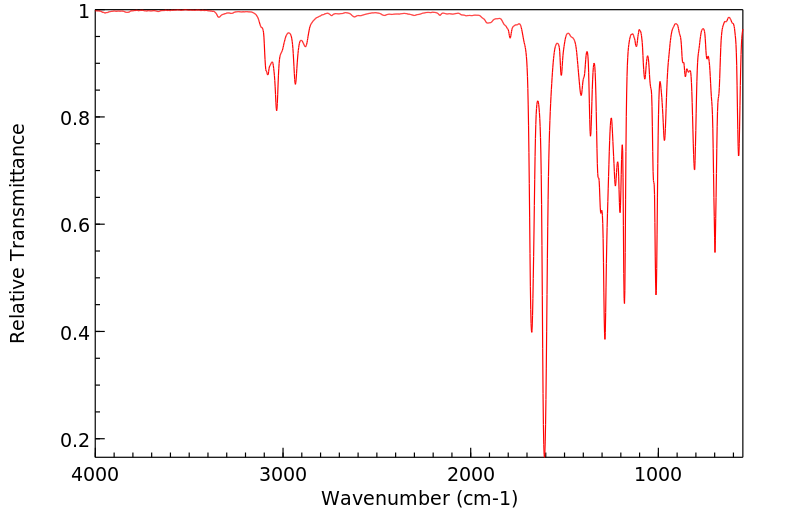
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫