N-(tert-butyl)-2,4,6-trimethylbenzamide | 63064-23-3
中文名称
——
中文别名
——
英文名称
N-(tert-butyl)-2,4,6-trimethylbenzamide
英文别名
N-t-Butyl-2,4,6-trimethylbenzamid;Benzamide, N-(1,1-dimethylethyl)-2,4,6-trimethyl-;N-tert-butyl-2,4,6-trimethylbenzamide
CAS
63064-23-3
化学式
C14H21NO
mdl
MFCD00704202
分子量
219.327
InChiKey
BHFNQUBVZGLPHL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:298.6±39.0 °C(Predicted)
-
密度:0.961±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:16
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:29.1
-
氢给体数:1
-
氢受体数:1
反应信息
-
作为反应物:描述:N-(tert-butyl)-2,4,6-trimethylbenzamide 在 sodium hydride 、 N-氟代双苯磺酰胺 作用下, 以 二氯甲烷 为溶剂, 反应 0.5h, 生成 N-(tert-butyl)-N-fluoro-2,4,6-trimethylbenzamide参考文献:名称:由氟酰胺定向远程苄基 C(sp3)–H 官能化实现的烯烃分子间 1,2-双官能化摘要:报道了一种铜催化的远程苄基 C-H 官能化策略,能够使烯烃与 2-甲基苯酰胺和亲核试剂(包括醇、吲哚、吡咯和固有氨基)进行 1,2-双官能化,其特点是氧化还原中性条件、精细的位点选择性、广泛的底物范围以及后期修饰生物活性分子的广泛应用。该反应通过以氮为中心的自由基生成、氢原子转移、烯烃上的苄基自由基加成、单电子氧化和碳阳离子亲电过程级联进行。虽然使用外部亲核试剂操纵三组分烯烃烷基烷氧基化和用 2-甲基苯酰胺进行烷基杂芳基化以得到二烷基醚、3-烷基吲哚和 3-烷基吡咯,f ][1,2]硫氮杂 1,1-二氧化物。DOI:10.1021/jacs.1c10053
-
作为产物:描述:参考文献:名称:有机合成中的磷-XI氨基酸和肽-XXI:磷腈二乙酯(DEPC)与羧酸的反应。羧酸酯和酰胺的新合成摘要:在三乙胺的存在下,二异氰酸二乙酯(DEPC)与羧酸反应,导致瞬态形成酰基氰,但在醇或胺的存在下,会生成羧酸酯或酰胺。DEPC对酰胺和肽的合成特别有效,并且在Young消旋试验中显示出令人满意的结果。DOI:10.1016/0040-4020(76)85134-4
文献信息
-
A Practical and General Amidation Method from Isocyanates Enabled by Flow Technology作者:Jason D. Williams、William J. Kerr、Stuart G. Leach、David M. LindsayDOI:10.1002/anie.201807393日期:2018.9.10The addition of carbon nucleophiles to isocyanates represents a conceptually flexible and efficient approach to the preparation of amides. This general synthetic strategy has, however, been relatively underutilized owing to narrow substrate tolerance and the requirement for less favourable reaction conditions. Herein, we disclose a high‐yielding, mass‐efficient, and scalable method with appreciable
-
Facile Synthesis of Sterically Hindered and Electron-Deficient Secondary Amides from Isocyanates作者:Gabriel Schäfer、Coraline Matthey、Jeffrey W. BodeDOI:10.1002/anie.201204481日期:2012.9.3The big easy: The direct coupling of Grignard reagents to isocyanates provides a facile and robust solution for the synthesis of sterically hindered and electron‐deficient secondary amides. The products are obtained in high yields without the need for excess reagents or chromatographic purification.
-
Synthesis of Secondary Amides from Thiocarbamates作者:Pieter Mampuys、Eelco Ruijter、Romano V. A. Orru、Bert U. W. MaesDOI:10.1021/acs.orglett.8b01654日期:2018.7.20The synthesis of secondary amides from readily accessible and bench-stable substituted S-phenyl thiocarbamates and Grignard reactants is reported. Oxidative workup allows recycling of the thiolate leaving group as diphenyl disulfide. Diphenyl disulfide can be transformed into S-phenyl benzenethiosulfonate, a reactant required for thiocarbamate synthesis. This amide synthesis is suitable for the preparation
-
INHIBITORS OF BRUTON'S TYROSINE KINASE申请人:Brotherton-Pleiss Christine E.公开号:US20130045965A1公开(公告)日:2013-02-21This application discloses compounds according to generic Formula I: wherein the variables are defined as described herein, and which inhibit Btk. The compounds disclosed herein are useful to modulate the activity of Btk and treat diseases associated with excessive Btk activity. The compounds are further useful to treat inflammatory and auto immune diseases associated with aberrant B-cell proliferation, such as rheumatoid arthritis. Also disclosed are compositions containing compounds of Formula I and at least one carrier, diluent or excipient.本申请公开了通用式I所示的化合物:其中变量如本文所述,并抑制Btk。本文所披露的化合物有助于调节Btk的活性并治疗与过度Btk活性相关的疾病。此外,这些化合物还有助于治疗与异常B细胞增殖相关的炎症和自身免疫性疾病,如类风湿性关节炎。还披露了含有通用式I化合物和至少一种载体、稀释剂或赋形剂的组合物。
-
Unusual reactivity of isocyanates with dioxo- and diimidomolybdenum(VI) complexes作者:Richard Lai、Sylvain Mabille、Alain Croux、Sylvie Le BotDOI:10.1016/s0277-5387(00)80213-2日期:1991.1Phenyl isocyanate reacts with dioxo- and diimidomolybdenum(VI) complexes such as Mo(O)2(mes)2 (1) (mes = mesityl = 2,4,6-C6H2Me3), Mo(mes)2CH2PBu3(O)2 (2), Mo(mes)CPBu3(mes)}(O)2(3) and Mo(NBu(t))2(mes)2 (4), selectively affording phenyl mesityl amide. This reaction proceeds via insertion into the Mo-mesityl bond, leading to an unstable oxazamolydenacycle which has been identified by C-13 NMR. This intermediate is probably hydrolysed with generation of the amide during chromatographic work-up.
表征谱图
-
氢谱1HNMR
-
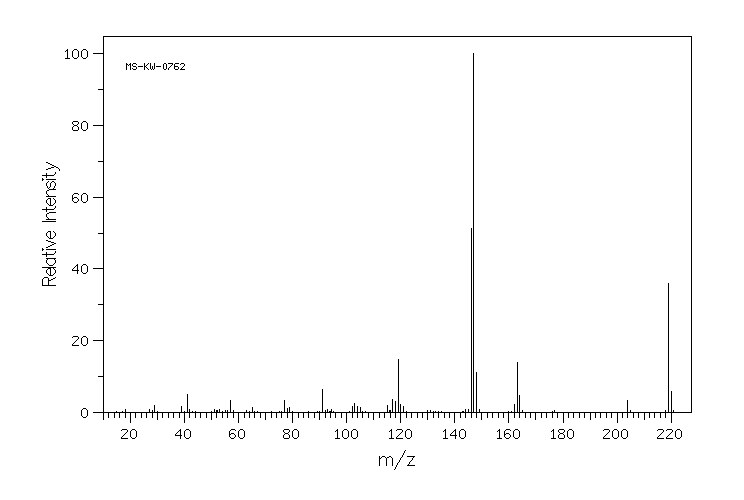
质谱MS
-
碳谱13CNMR
-
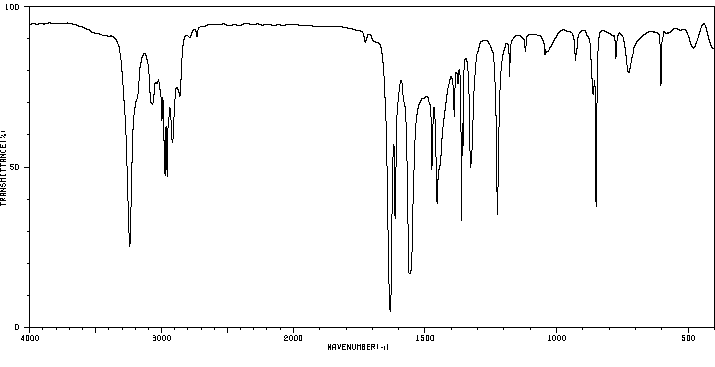
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫