5-acetyl-1-phenyl-pyrimidine-2,4,6-trione | 91974-06-0
中文名称
——
中文别名
——
英文名称
5-acetyl-1-phenyl-pyrimidine-2,4,6-trione
英文别名
5-acetyl-1-phenyl-barbituric acid;5-Acetyl-1-phenyl-barbitursaeure;1-Phenyl-5-acetyl-barbitursaeure;5-Acetyl-1-phenyl-1,3-diazinane-2,4,6-trione
CAS
91974-06-0
化学式
C12H10N2O4
mdl
——
分子量
246.222
InChiKey
QRFZVXIQSQOFMD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.5
-
重原子数:18
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:83.6
-
氢给体数:1
-
氢受体数:4
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-苯基巴比土酸 1-phenyl-pyrimidine-2,4,6-trione 15018-50-5 C10H8N2O3 204.185
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis and antifungal activity of substituted 2,4,6-pyrimidinetrione carbaldehyde hydrazones摘要:Opportunistic fungal infections caused by the Candida spp. are the most common human fungal infections, often resulting in severe systemic infections-a significant cause of morbidity and mortality in at-risk populations. Azole antifungals remain the mainstay of antifungal treatment for candidiasis, however development of clinical resistance to azoles by Candida spp. limits the drugs' efficacy and highlights the need for discovery of novel therapeutics. Recently, it has been reported that simple hydrazone derivatives have the capability to potentiate antifungal activities in vitro. Similarly, pyrimidinetrione analogs have long been explored by medicinal chemists as potential therapeutics, with more recent focus being on the potential for pyrimidinetrione antimicrobial activity. In this work, we present the synthesis of a class of novel hydrazone-pyrimidinetrione analogs using novel synthetic procedures. In addition, structure-activity relationship studies focusing on fungal growth inhibition were also performed against two clinically significant fungal pathogens. A number of derivatives, including phenylhydrazones of 5-acylpyrimidinetrione exhibited potent growth inhibition at or below 10 mu M with minimal mammalian cell toxicity. In addition, in vitro studies aimed at defining the mechanism of action of the most active analogs provide preliminary evidence that these compound decrease energy production and fungal cell respiration, making this class of analogs promising novel therapies, as they target pathways not targeted by currently available antifungals. (C) 2013 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2013.12.010
-
作为产物:描述:参考文献:名称:Ridi, Annali di Chimica, 1952, vol. 42, p. 25,29摘要:DOI:
文献信息
-
Preparation of 5-formyl- and 5-acetylbarbituric acids, including the corresponding Schiff bases and phenylhydrazones作者:Branko S Jursic、Donna M NeumannDOI:10.1016/s0040-4039(01)01830-5日期:2001.11effective, and high yield synthetic procedures for formylation and acetylation of barbituric acid derivatives is described. Generated 5-acylbarbituric acids are transformed into the corresponding Schiff bases in high yield condensation reactions with ω-aminoalkanoic acids and nitrophenylhydrazines.
-
US4260776A申请人:——公开号:US4260776A公开(公告)日:1981-04-07
表征谱图
-
氢谱1HNMR
-
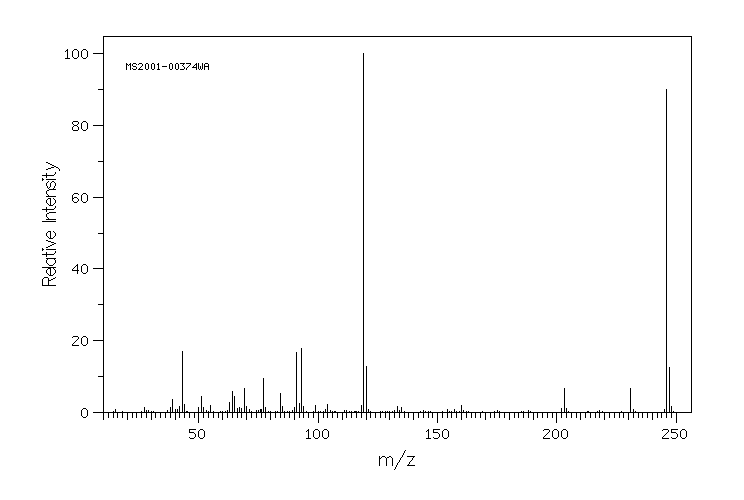
质谱MS
-
碳谱13CNMR
-
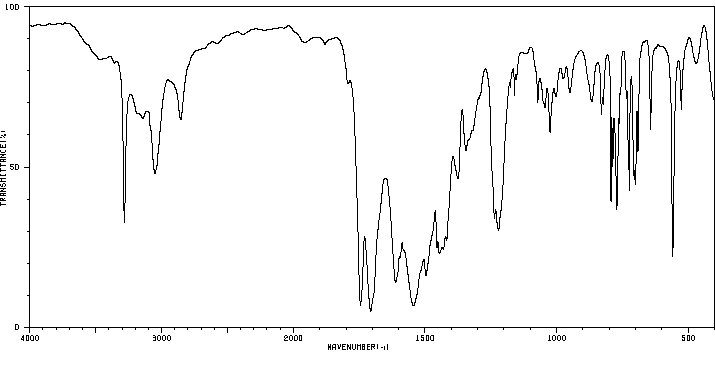
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3