trimethyl(3-methylphenyl)ammonium iodide | 33046-97-8
中文名称
——
中文别名
——
英文名称
trimethyl(3-methylphenyl)ammonium iodide
英文别名
trimethyl-m-tolylammonium iodide;tri-N-methyl-m-toluidinium; iodide;Tri-N-methyl-m-toluidinium; Jodid;Trimethyl-m-tolyl-ammonium iodide;m-Tolyltrimethylammonium iodide;trimethyl-(3-methylphenyl)azanium;iodide
CAS
33046-97-8
化学式
C10H16N*I
mdl
——
分子量
277.148
InChiKey
DFQLEGYTTZJIAC-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:177-178 °C
计算性质
-
辛醇/水分配系数(LogP):-0.8
-
重原子数:12
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:1
反应信息
-
作为反应物:描述:参考文献:名称:三烷基铵盐的降解:对甲基化和交叉偶联的影响摘要:已知三烷基铵(最显着的是N,N,N-三甲基苯铵)盐通过芳基和N-甲基均显示出双重反应性。因此,这些盐已广泛应用于交叉偶联,芳基醚化,氟放射性标记,相转移催化,超分子识别,聚合物设计和(最近)甲基化。然而,它们作为亲电甲基化试剂的应用仍未得到充分开发,并且缺乏对它们的芳基化与甲基化反应性的了解。本研究提出了N,N,N的机械降解分析。-三甲基苯胺盐,并突出了这一重要类别盐对合成应用的意义。在固相和固溶阶段的动力学降解研究已经深入了解了影响苯胺盐稳定性的物理和化学参数。盐降解的1 H NMR动力学分析表明,热降解为甲基碘和母体苯胺,与闭壳S N一致2位降解途径,而甲基碘是应用甲基化程序中的关键反应物种。此外,还研究了卤化物和非亲核抗衡离子对盐降解的影响,以及氘同位素和溶剂的影响。新的力学见解使人们能够研究三甲基苯胺盐在O-甲基化中的应用以及在改进的交叉偶联策略中的应用。最后,详细的计算研究有助于DOI:10.1039/d1sc00757b
-
作为产物:描述:参考文献:名称:MOLECULAR IMAGING AGENTS摘要:提供了放射标记的铵盐及其在分子影像中作为心肌灌注剂的用途。公开号:US20110293519A1
文献信息
-
Scalable, Metal- and Additive-Free, Photoinduced Borylation of Haloarenes and Quaternary Arylammonium Salts作者:Adelphe M. Mfuh、John D. Doyle、Bhuwan Chhetri、Hadi D. Arman、Oleg V. LarionovDOI:10.1021/jacs.6b01376日期:2016.3.9We report herein a simple, metal- and additive-free, photoinduced borylation of haloarenes, including electron-rich fluoroarenes, as well as arylammonium salts directly to boronic acids. This borylation method has a broad scope and functional group tolerance. We show that it can be further extended to boronic esters and carried out on gram scale as well as under flow conditions.
-
Molecular imaging agents申请人:Mishani Eyal公开号:US09205156B2公开(公告)日:2015-12-08Provided is radiolabeled ammonium salts and uses thereof as myocardial perfusion agents in molecular imaging.提供了放射性标记的铵盐及其在分子影像中作为心肌灌注剂的用途。
-
Selective Hofmann alkylation of aromatic-aliphatic diamines in the presence of carbon dioxide作者:Alexei G. Balybin、Yuri M. Panov、Ludmila V. Erkhova、Dmitry A. Lemenovskii、Dmitry P. Krut’koDOI:10.1016/j.mencom.2019.07.028日期:2019.7Selective Hofmann alkylation at arylamino group in carbon dioxide medium was demonstrated on model diamines containing aliphatic and aromatic primary amino groups, namely, 2-H2NC6H4CH2NH2, 3-H2NC6H4CH(Me)NH2, and 4-H2NC6H4CH2CH2NH2. Depending on the spatial factors, di- or trialkylarylammonium derivatives are selectively formed in amide solvents (DMF, DMA) without additional base.
-
Gavrilov, V. I.; Khusnutdinova, F. M., Journal of general chemistry of the USSR, 1987, vol. 57, p. 295 - 297作者:Gavrilov, V. I.、Khusnutdinova, F. M.DOI:——日期:——
-
Kokorev, G. I.; Yambushev, F. D.; Badrutdinov, Sh. Kh., Journal of general chemistry of the USSR, 1987, vol. 57, p. 762 - 764作者:Kokorev, G. I.、Yambushev, F. D.、Badrutdinov, Sh. Kh.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
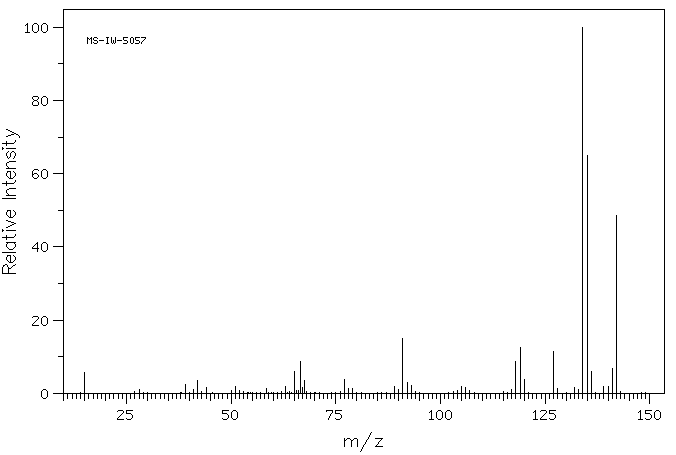
质谱MS
-
碳谱13CNMR
-
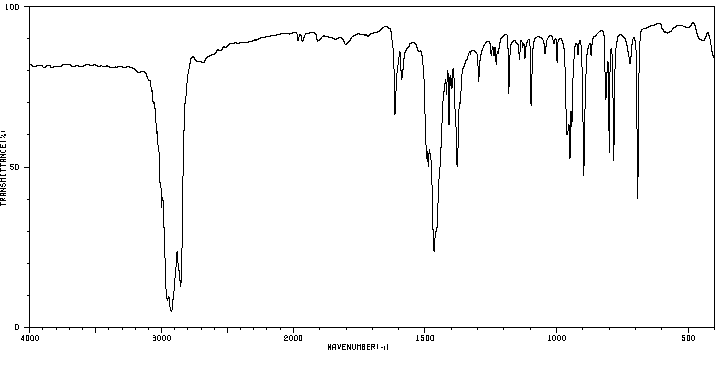
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫