N-(1-methylpiperidin-4-yl)-4-nitrobenzamide | 210643-97-3
中文名称
——
中文别名
——
英文名称
N-(1-methylpiperidin-4-yl)-4-nitrobenzamide
英文别名
N-(1-methyl-piperidin-4-yl)-4-nitro-benzamide
CAS
210643-97-3
化学式
C13H17N3O3
mdl
——
分子量
263.296
InChiKey
CDDSATAFEYARTB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:454.5±40.0 °C(Predicted)
-
密度:1.25±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:19
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.46
-
拓扑面积:78.2
-
氢给体数:1
-
氢受体数:4
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-amino-N-(1-methylpiperidin-4-yl)benzamide 50534-11-7 C13H19N3O 233.313
反应信息
-
作为反应物:描述:N-(1-methylpiperidin-4-yl)-4-nitrobenzamide 在 盐酸 、 tin(II) chloride dihdyrate 作用下, 以 1,4-二氧六环 、 乙醇 、 水 、 乙酸乙酯 为溶剂, 反应 16.0h, 生成 (R)-4-((8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,7,8-tetrahydropteridin-2-yl)amino)-N-(1-methylpiperidin-4-yl)benzamide参考文献:名称:BRD4 Structure–Activity Relationships of Dual PLK1 Kinase/BRD4 Bromodomain Inhibitor BI-2536摘要:A focused library of analogues of the dual PLK1 kinase/BRD4 bromodomain inhibitor BI-2536 was prepared and then analyzed for BRD4 and PLK1 inhibitory activities. Particularly, replacement of the cyclopentyl group with a 3-bromobenzyl moiety afforded the most potent BRD4 inhibitor of the series (39j) with a K-i = 8.7 nM, which was equipotent against PLK1. The superior affinity of 39j over the parental compound to BRD4 possibly derives from improved interactions with the WPF shelf. Meanwhile, substitution of the pyrimidine NH with an oxygen atom reversed the PLK1/BRD4 selectivity to convert BI-2536 into a BRD4-selective inhibitor, likely owing to the loss of a critical hydrogen bond in PLK1. We believe further fine-tuning will furnish a BRD4 "magic bullet" or an even more potent PLK1/BRD4 dual inhibitor toward the expansion and improved efficacy of the chemotherapy arsenal.DOI:10.1021/acsmedchemlett.5b00084
-
作为产物:描述:N-甲基-4-哌啶酮 在 palladium on carbon 甲酸铵 、 1-羟基苯并三唑 、 盐酸-N-乙基-Nˊ-(3-二甲氨基丙基)碳二亚胺 、 N,N-二异丙基乙胺 作用下, 以 四氢呋喃 、 甲醇 为溶剂, 生成 N-(1-methylpiperidin-4-yl)-4-nitrobenzamide参考文献:名称:[EN] 2, 4 -DIAMINOPYRIMIDINE DERIVATIVES AS PROTEIN KINASE INHIBITORS
[FR] DÉRIVÉS DE 2,4-DIAMINOPYRIMIDINE EN TANT QU'INHIBITEURS DE PROTÉINE KINASES摘要:本发明涉及一种新型的式(I)的嘧啶衍生物,其作为激酶抑制剂具有用途。更具体地,本发明涉及新型嘧啶化合物、其制备方法、含有这些化合物的药物组合物以及这些化合物在治疗增殖性疾病中的用途。公开号:WO2012059932A1
文献信息
-
[EN] PYRROLO[2,3-D]PYRIMIDINE DERIVATIVES AND THEIR USE IN THE TREATMENT OF CANCER<br/>[FR] DÉRIVÉS DE PYRROLO[2,3-D]PYRIMIDINE ET LEUR UTILISATION DANS LE TRAITEMENT DU CANCER申请人:SENTINEL ONCOLOGY LTD公开号:WO2021074251A1公开(公告)日:2021-04-22The invention provides compounds of the formula (1): or a salt or tautomer thereof wherein A, R1, R2, R3, R4, R5 and R6 are as defined herein. The compounds are inhibitors of Wee1 and/or PLK1 kinase and are envisaged to be useful in the treatment of cancers.这项发明提供了式(1)的化合物:或其盐或互变异构体,其中A、R1、R2、R3、R4、R5和R6如本文所定义。这些化合物是Wee1和/或PLK1激酶的抑制剂,预计在癌症治疗中有用。
-
SUBSTITUTED PYRIMIDODIAZEPINES申请人:Chen Shaoqing公开号:US20080234255A1公开(公告)日:2008-09-25The present invention provides PLK1 inhibitor compounds of formula I: Useful in the treatment or control of cell proliferative disorders, particularly oncological disorders. These compounds and formulations containing such compounds may be useful in the treatment or control of solid tumors, such as, for example, breast, colon, lung and prostate tumors and other oncological diseases such as non-Hodgkin's lymphomas.
-
Substituted pyrimidodiazepines申请人:Hoffman-La Roche Inc.公开号:US07517873B2公开(公告)日:2009-04-14The present invention provides PLK1 inhibitor compounds of formula I: Useful in the treatment or control of cell proliferative disorders, particularly oncological disorders. These compounds and formulations containing such compounds may be useful in the treatment or control of solid tumors, such as, for example, breast, colon, lung and prostate tumors and other oncological diseases such as non-Hodgkin's lymphomas.
-
Combining structure- and property-based optimization to identify selective FLT3-ITD inhibitors with good antitumor efficacy in AML cell inoculated mouse xenograft model作者:Hao Heng、Zhijie Wang、Hongmei Li、Yatian Huang、Qingyuan Lan、Xiaoxing Guo、Liang Zhang、Yanle Zhi、Jiongheng Cai、Tianren Qin、Li Xiang、Shuxian Wang、Yadong Chen、Tao Lu、Shuai LuDOI:10.1016/j.ejmech.2019.05.021日期:2019.8FLT3 mutation is among the most common genetic mutations in acute myeloid leukemia (AML), which is also related with poor overall survival and refractory in AML patients. Recently, FLT3 inhibitors have been approved for AML therapy. Herein, a series of new compounds with pyrazole amine scaffold was discovered, which showed potent inhibitory activity against FLT3-ITD and significant selectivity against both FLT3-ITD and AML cells expressing FLT3-ITD. Compound 46, possessing the most promising cellular activity, blocked the autophosphorylation of FLT3 pathway in MV4-11 cell line. Furthermore, the apoptosis and downregulation of P-STAT5 were also observed in tumor cells extracted from the MV411 cell xenografts model upon compound 46 treatment. Compound 46 was also metabolically stable in vitro and suppressed tumor growth significantly in MV4-11 xenografts model in vivo. Compound 46 showed no toxicity to the viscera of mice and caused no decrease in body weight of mice. In conclusion, the results of this study could provide valuable insights into discovery of new FLT3 inhibitors, and compound 46 was worthy of further development as potential drug candidate to treat AML (C) 2019 Elsevier Masson SAS. All rights reserved.
-
Synthesis of 1-methyl-4-(N-aroyl)-piperidinamides with anti-inflammatory and analgesic activities作者:Amedeo Pau、Gianpiero Boatto、Riccardo Cerri、Francesco Palagiano、Walter Filippelli、Giuseppe Falcone、Enrico LampaDOI:10.1016/s0014-827x(98)00016-0日期:1998.3Two series of 1-methyl-4-(N-aroyl)-piperidinamides were synthesized and evaluated for their anti-inflammatory and analgesic properties, as well as for their gastrointestinal irritation liability. A non-aromatic derivative, 1-methyl-4-(N-cyclohexanoyl)-piperidinamide, was synthe sized and evaluated in order to obtain a more exhaustive knowledge of the structure-activity relationship. (C) 1998 Elsevier Science S.A. All rights reserved.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
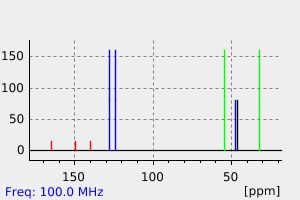
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫