5-硝基-2-(3-吡啶基)苯并咪唑 | 145861-59-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:242-244℃
-
沸点:518.9±56.0 °C(Predicted)
-
密度:1.451±0.06 g/cm3(Predicted)
-
溶解度:>36 [ug/mL]
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:18
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:87.4
-
氢给体数:1
-
氢受体数:4
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-(3-吡啶基)苯并咪唑 2-(3-pyridyl)-1H-benzimidazole 1137-67-3 C12H9N3 195.224 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-吡啶-3-基-1H-苯并咪唑-5-胺 5-amino-2-(β-pyridyl)benzimidazoles 1571-99-9 C12H10N4 210.238
反应信息
-
作为反应物:描述:参考文献:名称:2-取代的苯并咪唑的化学。1. 5-氨基-2-甲基(芳基,芳烷基,吡啶基)苯并咪唑摘要:DOI:10.1007/bf02269539
-
作为产物:描述:参考文献:名称:2-取代的苯并咪唑的化学。1. 5-氨基-2-甲基(芳基,芳烷基,吡啶基)苯并咪唑摘要:DOI:10.1007/bf02269539
文献信息
-
Synthesis of Pyridinyl-benzo[d]imidazole/Pyridinyl-benzo[d]thiazole Derivatives and their Yeast Glucose Uptake Activity In Vitro作者:Momin Khan、Riaz Ahmad、Gauhar Rehman、Naeem Gul、Sana Shah、Uzma Salar、Shahnaz Perveen、Khalid Mohammed KhanDOI:10.2174/1570180815666181004102209日期:2019.9.11
Background: Diabetes is the primary cause of fatality and disability all over the world, in recent past, we have reported various classes of compounds as anti-glycating agents and we have also reported benzimidazole and benzothiazole derivatives as a potential class of anti-glycating agents. This encouraged us to evaluate the pyridinyl benzimidazole/pyridinyl benzothiazole derivatives 1-27 for yeast glucose uptake activity.
Methods: In the present study, an equimolar mixture of pyridine carboxaldehyde derivatives (1 mmol) and sodium metabisulphite (1 mmol) in DMF (10 mL) was stirred for 10 to 15 min, followed by addition of o-phenylene diamine/2-aminothiophenol (1 mmol) into it and refluxed for 3 h. The progress of the reaction was monitored by TLC. After completion, the reaction mixture was poured into crushed ice. Precipitates were formed which were collected by filtration to produce compounds 1-27 in good yields. Recrystallization from methanol yielded pure crystals.
Results: Our present study showed that all compounds showed a varying degree of yeast glucose uptake activity in the range IC50 = 36.43-272.20 µM, compared to standard metronidazole (IC50 = 41.86 ± 0.09 µM). Compounds 5 (IC50 = 38.14 ± 0.17 µM), 6 (IC50 = 40.23 ± 0.20 µM), and 7 (IC50 = 36.43 ± 0.02 µM) showed an excellent yeast glucose uptake activity better than the standard.
Conclusion: Pyridinyl benzimidazole/pyridinyl benzothiazole derivatives 1-27 were synthesized, structurally characterized, and evaluated for in vitro yeast glucose uptake activity. Compounds 5 (IC50 = 38.14 ± 0.17 µM), 6 (IC50 = 40.23 ± 0.20 µM), and 7 (IC50 = 36.43 ± 0.02 µM) demonstrated potent yeast glucose uptake activity as compared to standard metronidazole (IC50 = 41.86 ± 0.09 µM). This study identified a number of potential lead molecules which can be helpful in lowering the blood glucose level in hyperglycemia.
背景:糖尿病是全球致死和致残的主要原因,最近,我们已报告各种类别的化合物作为抗糖基化剂,并且我们还报告苯并咪唑和苯并噻唑衍生物作为一种潜在的抗糖基化剂类。这激励我们评估吡啶基苯并咪唑/吡啶基苯并噻唑衍生物1-27对酵母葡萄糖摄取活性的作用。 方法:在本研究中,将吡啶羧醛衍生物(1 mmol)和亚硫酸钠(1 mmol)在DMF(10 mL)中等摩尔混合物搅拌10至15分钟,然后加入邻苯二胺/2-氨基硫酚(1 mmol)并回流3小时。通过薄层色谱监测反应进展。反应完成后,将反应混合物倒入碎冰中。形成沉淀,通过过滤收集,产生产率良好的化合物1-27。用甲醇再结晶得到纯晶体。 结果:我们的研究表明,所有化合物在酵母葡萄糖摄取活性范围内显示出不同程度的活性,IC50 = 36.43-272.20 µM,与标准甲硝唑(IC50 = 41.86 ± 0.09 µM)相比。化合物5(IC50 = 38.14 ± 0.17 µM)、6(IC50 = 40.23 ± 0.20 µM)和7(IC50 = 36.43 ± 0.02 µM)显示出优异的酵母葡萄糖摄取活性,优于标准物质。 结论:合成了吡啶基苯并咪唑/吡啶基苯并噻唑衍生物1-27,进行了结构表征,并评估了体外酵母葡萄糖摄取活性。化合物5(IC50 = 38.14 ± 0.17 µM)、6(IC50 = 40.23 ± 0.20 µM)和7(IC50 = 36.43 ± 0.02 µM)表现出强大的酵母葡萄糖摄取活性,与标准甲硝唑(IC50 = 41.86 ± 0.09 µM)相比。该研究确定了一系列潜在的先导分子,有助于降低高血糖水平。 -
Synthesis of 2-(1-methyl-1,2,5,6-tetrahydropyridin-3-yl)benzimidazoles作者:Myung Hee Jung、Jung Mee Park、Ihl-Young Choi Lee、Mija AhnDOI:10.1002/jhet.5570400104日期:2003.1A useful approach for the synthesis of pharmacologically active tetrahydropyridinylbenzimidazoles is described. 2-Pyridin-3-ylbenzimidazoles 3a-d have been synthesized by condensation of 3-pyridinecarbox-aldehyde 1 with substituted 1,2-phenylenediamines 2a-d following oxidative cyclization with iodobenzene diacetate. Methylation of 3a-d with iodomethane and potassium hydroxide, subsequent formation
-
Design, synthesis and biological evaluation of some novel benzimidazole derivatives for their potential anticonvulsant activity作者:Priyal Jain、Prakash Kumar Sharma、Harish Rajak、Rajesh Singh Pawar、Umesh Kumar Patil、Pradeep Kumar SingourDOI:10.1007/s12272-010-0701-8日期:2010.7equation indicated that the binding affinity is strongly dependent upon the thermodynamic properties (CDE, DDE and PC). Correlation between these properties and anticonvulsant activity was used to synthesize compounds possessing potent anticonvulsant activity. Most of the compounds showed an ability to inhibit the maximum electroshock (MES) and pentylenetetrazole (PTZ)-induced convulsions. Compound 1A, i选择性GABAA受体配体在临床上被广泛用于减少惊厥的发生。因此,人们对开发对 GABAA 受体表现出高选择性和高亲和力的新苯并咪唑衍生物有着浓厚的兴趣。为了设计对 GABAA/BZd 受体复合物具有增强结合亲和力的新化学实体,我们对苯并三嗪衍生物进行了 QSAR 研究。我们研究了 28 种有效的 GABAA 受体配体;苯并三嗪衍生物,使用各种经过测试的物理化学、空间、电子和热力学描述符的组合来确定结合亲和力和结构特征之间的定量相关性。开发和验证的最终模型显示出良好的相关性和预测能力,相关系数平方 (r2) 为 0.954。该方程表明结合亲和力强烈依赖于热力学特性(CDE、DDE 和 PC)。这些特性与抗惊厥活性之间的相关性用于合成具有强抗惊厥活性的化合物。大多数化合物显示出抑制最大电休克 (MES) 和戊四唑 (PTZ) 诱发的惊厥的能力。化合物1A,即2-(4-氯-苯基)-5-硝基-1H
-
IMIDAZOLE DERIVATIVES HAVING ARYL PIPERIDINE SUBSTITUENT, METHOD FOR PREPARATION THEREOF AND PHARMACEUTICAL COMPOSITIONS CONTAINING SAME申请人:Suh Jee Hee公开号:US20100145054A1公开(公告)日:2010-06-10The present invention is directed to a novel imidazole derivative having an aryl piperidine substituent of formula (I) and a method for preparation thereof, and a pharmaceutical composition containing said imidazole derivative as an active ingredient for preventing or treating a MCH (melanine-concentrating hormone)-related disease.
-
Synthesis and evaluation of novel bacterial rRNA-binding benzimidazoles by mass spectrometry作者:Yun He、Jun Yang、Baogen Wu、Dale Robinson、Kelly Sprankle、Pei-Pei Kung、Kristin Lowery、V. Mohan、Steve Hofstadler、Eric E. Swayze、Rich GriffeyDOI:10.1016/j.bmcl.2003.11.031日期:2004.2A series of novel benzimidazoles were efficiently synthesized using both solution- and solid-phase chemistry. These compounds were found to bind to the bacterial 16S ribosomal RNA A-site with micromolar affinities using unique mass spectrometry-based assays. (C) 2003 Elsevier Ltd. All rights reserved.
表征谱图
-
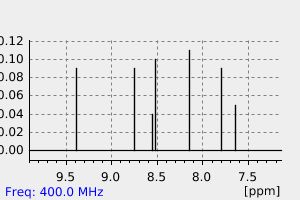
氢谱1HNMR
-
质谱MS
-
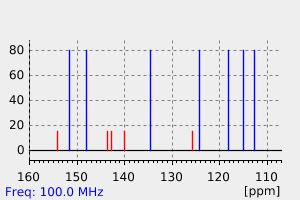
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息