N,N-Diisopropyl-N'-formyl-hydrazin | 3298-51-9
中文名称
——
中文别名
——
英文名称
N,N-Diisopropyl-N'-formyl-hydrazin
英文别名
N-[di(propan-2-yl)amino]formamide
CAS
3298-51-9
化学式
C7H16N2O
mdl
——
分子量
144.217
InChiKey
RCYKKVZDPPOLKZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:10
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.86
-
拓扑面积:32.3
-
氢给体数:1
-
氢受体数:2
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N,N-二异丙基肼 N,N-diisopropylhydrazine 921-14-2 C6H16N2 116.206
反应信息
-
作为反应物:描述:N,N-Diisopropyl-N'-formyl-hydrazin 在 lithium aluminium tetrahydride 作用下, 以 乙醚 为溶剂, 反应 5.0h, 生成 1,1-diisopropyl-2-methylhydrazine参考文献:名称:通过动态NMR-28进行的构象研究:一些二烷基,三烷基和四烷基肼的立体构型摘要:低温NMR光谱使我们可以“冻结”许多二,三和四烷基肼中的某些内部运动,并测量相应的活化自由能。特别地,发现四甲基肼(Me 2 N-NMe 2)在-150°下具有两对非对位甲基:这是由于在该温度下N反转和N,N旋转均很慢的事实并且采用了gauche构象。观察到的势垒(6.0kcal mol -1)归因于N,N-旋转,由于N-反转引起的势垒较高,并且在伴随快速旋转的情况下不能通过NMR测量。在其他情况下,尤其是Pr i MeN-NH 2,Me 2N-NPr i 2和Pr i 2 N-NHMe,检测到两个不同的运动(反转和旋转)。在Me 2 N-NHMe的情况下,还可以观察到由在低温下变为手性的胺氮诱导的氮键合甲基(Me 2 N)的等时性行为的第一个例子。DOI:10.1016/s0040-4020(01)96566-4
-
作为产物:参考文献:名称:Walter,W.; Reubke,K.-J., Chemische Berichte, 1970, vol. 103, p. 2197 - 2207摘要:DOI:
文献信息
-
Highly Enantioselective Transfer Hydrogenation of Ketones with Chiral (NH)<sub>2</sub>P<sub>2</sub>Macrocyclic Iron(II) Complexes作者:Raphael Bigler、Raffael Huber、Antonio MezzettiDOI:10.1002/anie.201501807日期:2015.4.20Bis(isonitrile) iron(II) complexes bearing a C2‐symmetric diamino (NH)2P2 macrocyclic ligand efficiently catalyze the hydrogenation of polar bonds of a broad scope of substrates (ketones, enones, and imines) in high yield (up to 99.5 %), excellent enantioselectivity (up to 99 % ee), and with low catalyst loading (generally 0.1 mol %). The catalyst can be easily tuned by modifying the substituents of
-
Bredereck,H. et al., Justus Liebigs Annalen der Chemie, 1965, vol. 686, p. 92 - 101作者:Bredereck,H. et al.DOI:——日期:——
表征谱图
-
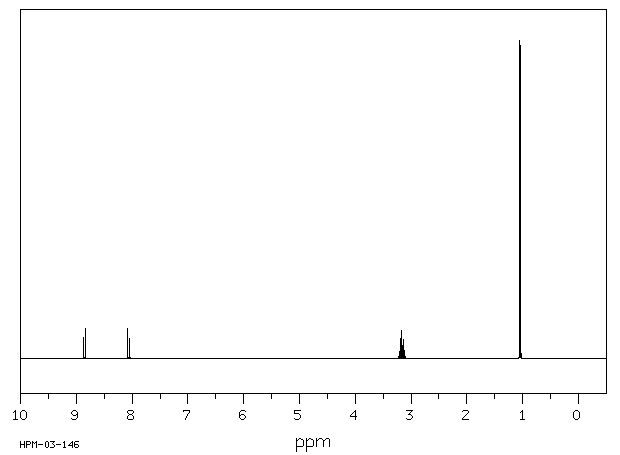
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸