苯乙醛,2-碘- | 109347-41-3
中文名称
苯乙醛,2-碘-
中文别名
——
英文名称
2-(2-iodophenyl)acetaldehyde
英文别名
(2-iodophenyl)acetaldehyde
CAS
109347-41-3
化学式
C8H7IO
mdl
——
分子量
246.047
InChiKey
ZMOIBLKIUKDUFP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:10
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-碘苯乙酸 o-iodophenylacetic acid 18698-96-9 C8H7IO2 262.047 2-(2-碘苯基)-1-乙醇 2-(2-iodophenyl)ethan-1-ol 26059-40-5 C8H9IO 248.063 2-(2-碘苯基)乙酸甲酯 methyl (2-iodophenyl)acetate 66370-75-0 C9H9IO2 276.074
反应信息
-
作为反应物:描述:苯乙醛,2-碘- 在 zinc(II) chloride 作用下, 以 四氢呋喃 、 二氯甲烷 为溶剂, 生成 (2R)-N-(2,3,4,6-tetra-O-pivaloyl-β-D-galactopyranosyl)-2-(2-iodobenzyl)-5,6-didehydro-piperidin-4-one参考文献:名称:Palladium-catalysed C–C coupling reactions in the enantioselective synthesis of 2,4-disubstituted 4,5-dehydropiperidines using galactosylamine as a stereodifferentiating auxiliary摘要:Stereoselective synthesis of enantiomerically pure 2,4-disubstituted piperidine derivatives, which are considered interesting pharmacophoric structures, was achieved starting with a tandem Mannich-Michael reaction sequence on O-pivaloylated N-galactosyl aldimines. Subsequent conversion of the thus formed 2-substituted dehydropiperidinones into the corresponding enol tri-flates was carried out by conjugate hydride addition and trapping the enolate with N,N-bis(trifluoromethanesulfonyl)aniline. Their Suzuki-Miyaura coupling with aryl and heteroaryl boronic acids was performed under, conditions compatible with the carbohydrate structure, in particular, with the sensitive N-glycosidic bond. (C) 2004 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetasy.2004.11.074
-
作为产物:参考文献:名称:分子内还原镍催化立体选择性合成鬼臼木核摘要:镍催化还原还原级联的非对映体控制的THN [2,3-c]呋喃。温和的反应条件导致了宽泛的官能团的耐受性,这些官能团几乎可以以良好的产率置于该骨架的每个位置。还提出了在串联环化偶联过程中观察到的反式或顺式融合选择性的构象控制。DOI:10.1039/c8cc00001h
文献信息
-
Synthesis of Indolines via a Domino Cu-Catalyzed Amidation/Cyclization Reaction作者:Ana Minatti、Stephen L. BuchwaldDOI:10.1021/ol8008792日期:2008.7.3A highly efficient one-pot procedure for the synthesis of indolines and their homologues based on a domino Cu-catalyzed amidation/nucleophilic substitution reaction has been developed. Substituted 2-iodophenethyl mesylates and related compounds afforded the corresponding products in excellent yields. No erosion of optical purity was observed when transforming enantiomerically pure mesylates under the
-
[EN] KRAS G12C INHIBITORS<br/>[FR] INHIBITEURS DE KRAS G12C申请人:MIRATI THERAPEUTICS INC公开号:WO2020146613A1公开(公告)日:2020-07-16The present invention relates to compounds that inhibit KRas G12C. In particular, the present invention relates to compounds that irreversibly inhibit the activity of KRas G12C, pharmaceutical compositions comprising the compounds and methods of use therefor.本发明涉及抑制KRas G12C的化合物。特别是,本发明涉及不可逆地抑制KRas G12C活性的化合物,包括含有这些化合物的药物组合物及其用途方法。
-
Synthesis of 3,4-Fused Tricyclic Indoles through Cascade Carbopalladation and C–H Amination: Development and Total Synthesis of Rucaparib作者:Cang Cheng、Xiang Zuo、Dongdong Tu、Bin Wan、Yanghui ZhangDOI:10.1021/acs.orglett.0c01513日期:2020.7.23,4-Fused tricyclic indole scaffolds are ubiquitous in bioactive natural products and pharmaceuticals. A new protocol for the synthesis of 3,4-fused tricyclic indoles has been developed through cascade carbopalladation and C–H amination with N,N-di-tert-butyldiaziridinone. The protocol allows access to a range of 3,4-fused tricyclic indoles, including those containing various linkers and fused with
-
Synthesis of Substituted Naphthalenes and Carbazoles by the Palladium-Catalyzed Annulation of Internal Alkynes作者:Qinhua Huang、Richard C. LarockDOI:10.1021/jo034449x日期:2003.9.1An efficient synthesis of highly substituted naphthalenes has been developed by the palladium-catalyzed annulation of a variety of internal alkynes, in which two new carbon-carbon bonds are formed in a single step under relatively mild reaction conditions. This method has also been used to synthesize carbazoles, although a higher reaction temperature is necessary. The process involves arylpalladation
-
Nickel-Mediated Inter- and Intramolecular Reductive Cross-Coupling of Unactivated Alkyl Bromides and Aryl Iodides at Room Temperature作者:Chang-Song Yan、Yu Peng、Xiao-Bo Xu、Ya-Wen WangDOI:10.1002/chem.201200190日期:2012.5.7A nickel‐mediated intermolecular reductive cross‐coupling reaction of unactivated alkyl bromides and aryl iodides at room temperature has been developed and successfully extended to less explored intramolecular versions and tandem cyclization‐intermolecular cross‐coupling. Highly stereoselective (or stereospecific) synthesis of linear‐fused perhydrofuro[2,3‐b]furan (pyran) and spiroketal skeletons
表征谱图
-
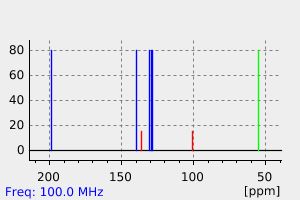
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫