9-癸基蒽 | 76881-11-3
中文名称
9-癸基蒽
中文别名
——
英文名称
9-n-decylanthracene
英文别名
9-decylanthracene
CAS
76881-11-3
化学式
C24H30
mdl
——
分子量
318.502
InChiKey
QDYQFGPPJYKEMT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):9.8
-
重原子数:24
-
可旋转键数:9
-
环数:3.0
-
sp3杂化的碳原子比例:0.42
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:4-十五烷基吡啶在Au(111)电极上扩散的光谱电化学研究†摘要:借助于电化学和光谱技术研究了4-十五烷基吡啶(C15-4-Py)在Au(111)电极上的吸附。电化学研究表明,C15-4-Py膜以接近零电荷(pzc)的电势扩散到金属表面,并以足够负的电势从电极表面解吸。C15–4-Py的吸附特性类似于吡啶分子在Au(111)电极上的吸附,表明膜的性能在很大程度上取决于吡啶部分与金属表面之间的相互作用。加入25摩尔%的10-癸基-9- [2-(4-吡啶基)乙基]蒽(DPEA),一种染料分子,C15–4-Py膜的特性仅轻微改变了其性能,但允许使用光谱方法研究膜的性能。荧光光谱和光散射实验被用来研究表面活性剂分子的潜在诱导的解吸和吸附。使用光谱电化学技术,我们已经证明了膜的解吸及其再吸收涉及在表面下区域形成胶束以及将胶束散布到电极表面上。已经提出了将不溶性表面活性剂铺展到电极表面上的一般机理。使用光谱电化学技术,我们已经证明了膜的解吸及其再吸收涉及在表面下区域DOI:10.1002/ijch.199700024
-
作为产物:参考文献:名称:具有不寻常头群拓扑结构的新型两亲物家族的合成和聚集特性摘要:已经合成了一个新的两亲物家族,它们源自具有不寻常拓扑结构的刚性二羧酸头基单元。这些分子在水溶液中的聚集已经通过 1 H NMR 和染料溶解方法进行了检查。两种聚集模式似乎在这组两亲分子中起作用,一种由头部决定,另一种由柔性尾部决定。当尾部很短或不存在时,前一种模式占优势,当尾部含有六个或更多非极性原子时,后者占优势。后一种模式看似是典型的胶束化过程,但前者的合作性较差DOI:10.1021/ja00036a048
文献信息
-
Synthesis of Conjugated Polyenes with Alkylanthryl and N-Alkylpyridinium Terminal Groups作者:Franz Effenberger、Stephan Heid、Rotraut Merkle、Peter ZimmermannDOI:10.1055/s-1995-4059日期:1995.9The synthesis of polyenes with 3 or 5 conjugated double bonds bearing alkylanthryl and alkylpyridinium terminal groups is described. Alkyl chains of suitable length were introduced in both the anthracene and/or pyridine moiety to reduce the solubility in water thus improving the ability to form Langmuir-Blodgett films.
-
Calorimetric and quantum chemical studies of some photodimers of anthracenes作者:Stefan Grimme、Sigrid D. Peyerimhoff、Henri Bouas-Laurent、Jean-Pierre Desvergne、Hans-Dieter Becker、Stefan M. Sarge、Herbert DreeskampDOI:10.1039/a900965e日期:——By differential scanning calorimetry (DSC) the reaction enthalpies of the thermal splitting of the following photochemically produced dimers of meso-substituted anthracenes in fluid solutions were determined: the head-to-tail (ht) dimers of 9-decylanthracene (DEA) and 9-decyloxyanthracene (DOA) in hydrocarbon solvents, and the head-to-head (hh) dimer of 9-methoxyanthracene (MOA) and the mixed hh dimer of 9-methoxyanthracene and 9-methoxy-10-methylanthracene (MMOA) in chlorinated solvents. The molar reaction enthalpy (in kJ mol-1) is found to be lower for the ht dimers (-28 kJ mol-1, DEA and -29 kJ mol-1, DOA) than for the hh dimers (-43 kJ mol-1, MOA and -44 kJ mol-1, MMOA) with an estimated precision of ±3 kJ mol-1 each. Quantum mechanical computations give energy differences for the thermal splitting of dianthracene and the two dimers of 9-methoxyanthracene in reasonable agreement with the reaction enthalpies observed. An analysis of the change in bond energies caused by the dimerization reveals that the relative stability of the dimer is mainly caused by a large increase in bond strength of the lateral benzene rings on dimerization.通过差示扫描量热法(DSC)测定了下列光化学生成的中取代蒽二聚体在流体溶液中的热裂解反应焓:烃类溶剂中 9-癸基蒽(DEA)和 9-癸氧基蒽(DOA)的头尾(ht)二聚体,以及氯化溶剂中 9-甲氧基蒽(MOA)的头顶(hh)二聚体和 9-甲氧基蒽和 9-甲氧基-10-甲基蒽(MMOA)的混合 hh 二聚体。发现 ht 二聚体的摩尔反应焓(kJ mol-1)(-28 kJ mol-1,DEA 和 -29 kJ mol-1,DOA)低于 hh 二聚体(-43 kJ mol-1,MOA 和 -44 kJ mol-1,MMOA),估计精度分别为 ±3 kJ mol-1。量子力学计算得出的二蒽和 9-甲氧基蒽的两种二聚体热裂解的能量差异与观察到的反应焓基本一致。对二聚化引起的键能变化的分析表明,二聚物的相对稳定性主要是由二聚化时侧向苯环键强度的大幅增加引起的。
-
Synthesis and characterization of anthracene-clustering dendrimers: observation of fluorescence resonance energy transfer in the multichromophoric system作者:Masaki Takahashi、Hironao Morimoto、Yousuke Suzuki、Tomoya Odagi、Mitsuji Yamashita、Hideki KawaiDOI:10.1016/j.tet.2004.09.096日期:2004.12A series of anthracene-clustering dendrimers bearing various aliphatic substituents at the terminal positions were synthesized using a direct coupling strategy. A remarkable effect of the side chains was imparted to chemical properties of the dendrimers such as drastically increased solubility. Although the multibranched anthracene arrays in the dendritic architectures exhibited no cooperativity in terms of the absorption feature and behaved as single chromophoric systems, investigations focusing on fluorescence properties revealed that a type of cooperativity was present as expressed in the reduced quantum yields of fluorescence. An alternative approach utilizing time-resolved fluorescence decay measurements clearly demonstrated that the most reasonable mechanism of the cooperative action should involve two discernible channels of intramolecular fluorescence resonance energy transfer (FRET) occurring from one chromophore to the others within and across junctions of the branching units. (C) 2004 Elsevier Ltd. All rights reserved.
表征谱图
-
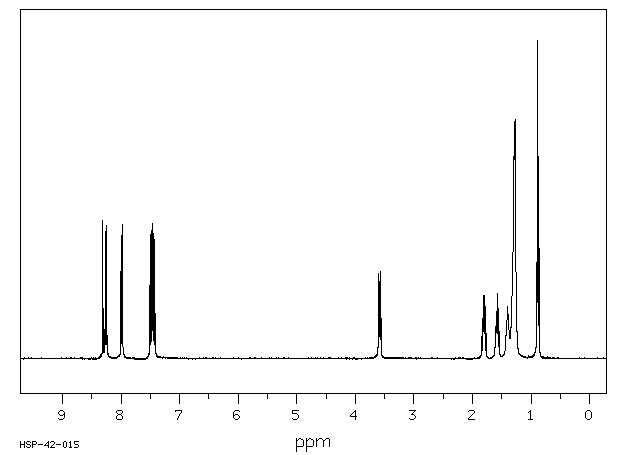
氢谱1HNMR
-
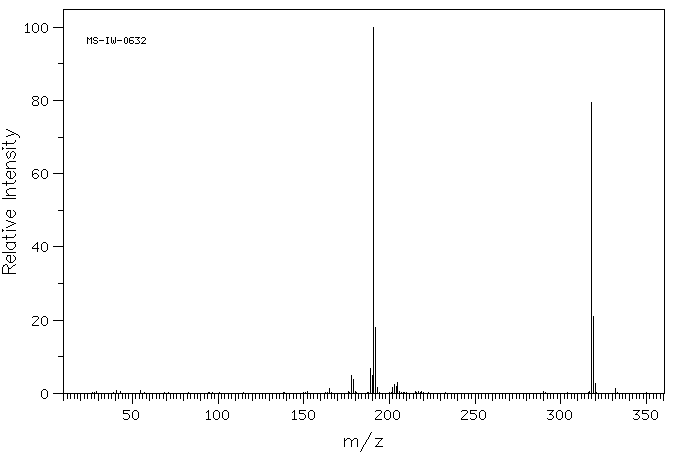
质谱MS
-
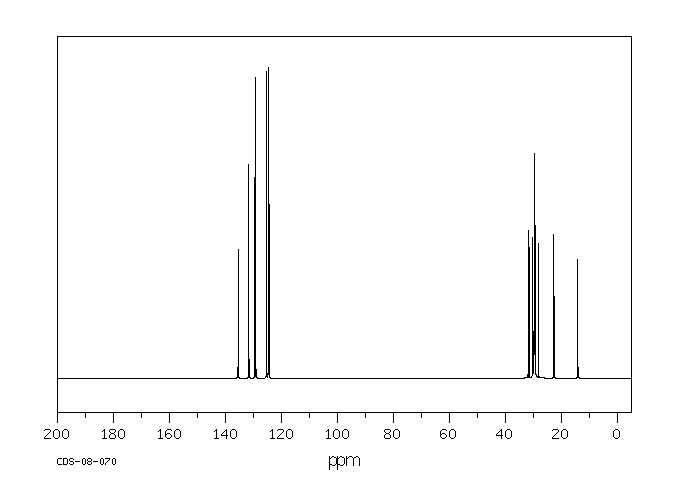
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62