苯基氨基甲酸丙酯 | 5532-90-1
中文名称
苯基氨基甲酸丙酯
中文别名
——
英文名称
n-propyl N-phenylcarbamate
英文别名
propyl phenylcarbamate;propyl N-phenylcarbamate;1-propyl phenylcarbamate;N-phenyl carbamic acid n-propyl ester
CAS
5532-90-1
化学式
C10H13NO2
mdl
MFCD00027110
分子量
179.219
InChiKey
QDZXCXBFZLLQFT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:57.85°C
-
沸点:311.75°C (rough estimate)
-
密度:1.1248 (rough estimate)
-
保留指数:1491;1493;1493;1490;1483;1503;1514
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:13
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:38.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2924299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-苯基香豆甲酯 N-phenyl methyl carbamate 2603-10-3 C8H9NO2 151.165
反应信息
-
作为反应物:参考文献:名称:A new and efficient catalytic method for synthesizing isocyanates from carbamates摘要:Operationally simple, recyclable and environmentally friendly montmorillonite efficiently catalyses dealcoholysis of a wide range of mono- and dicarbamates to isocyanates. (C) 2002 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0040-4039(02)00094-1
-
作为产物:描述:参考文献:名称:Metal free oxidative coupling of aryl formamides with alcohols for the synthesis of carbamates摘要:在无金属条件下,已开发出一种新方法,可将N-芳基甲酰胺直接转化为相应的N-芳基氨基甲酸酯,使用醇和过氧化碘作为氧化剂。据推测,该反应通过异氰酸酯中间体的形成进行。DOI:10.1039/c4ob00066h
文献信息
-
Fe<sub>3</sub>O<sub>4</sub>@SiO<sub>2</sub>/Schiff base/Pd complex as an efficient heterogeneous and recyclable nanocatalyst for chemoselective N-arylation of O-alkyl primary carbamates作者:A. R. Sardarian、M. Zangiabadi、I. Dindarloo InalooDOI:10.1039/c6ra17268g日期:——
An Fe3O4@SiO2/Schiff base/Pd complex as an efficient, heterogeneous magnetically recoverable and reusable catalyst for the
N -arylation ofO -alkyl primary carbamates. -
Nickel‐Catalyzed Synthesis of <i>N</i> ‐(Hetero)aryl Carbamates from Cyanate Salts and Phenols Activated with Cyanuric Chloride作者:Iman Dindarloo Inaloo、Mohsen Esmaeilpour、Sahar Majnooni、Ali Reza OveisiDOI:10.1002/cctc.202000876日期:2020.11.5one‐pot synthesis of N‐(hetero)aryl carbamates through the reaction between alcohols and in‐situ produced (hetero)aryl isocyanates in the presence of a nickel catalyst. The phenolic C−O bond was activated via the reaction of phenol with cyanuric chloride (2,4,6‐trichloro‐1,3,5‐triazine (TCT)) as an inexpensive and readily available reagent. This strategy provides practical access to N‐(hetero)aryl carbamates
-
(4-Arylsulfamoyl)phenylcarbamic acid esters: I. Synthesis and activity against herpes viruses作者:V. I. Krutikov、A. V. Erkin、V. V. Tets、A. A. ShmarovDOI:10.1134/s1070363216070069日期:2016.7Aiming to modify the biological activity of sulfonamides, a number of alkyl (4-arylsulfamoyl)- phenylcarbamates were prepared in 50–70% yield. Biological screening showed that the target compounds possessed a high activity against herpes viruses as well as a traditional antibiotic one.
-
Halogenation Using Quaternary Ammonium Polyhalides. IX. One-Step Syntheses of Acylureas and Carbamates from Amides by Use of Tetrabutylammonium Tribromide and DBU作者:Shizuo Fujisaki、Kazushi Tomiyasu、Akiko Nishida、Shoji KajigaeshiDOI:10.1246/bcsj.61.1401日期:1988.4The reaction of amides with tetrabutylammonium tribromide (TBA Br3) (0.5 equiv) and DBU (one equiv) in dichloromethane at room temperature gave N-substituted acylureas in fairly good yields. In the...
-
Synthesis of Carbonates and Related Compounds from Carbon Dioxide via Methanesulfonyl Carbonates作者:Mark O. Bratt、Paul C. TaylorDOI:10.1021/jo026753g日期:2003.7.1Carbonate anions resulting from reaction of primary or secondary alcohols with carbon dioxide, when added to methanesulfonic anhydride in cooled acetonitrile solution, yield methanesulfonyl carbonates, a new class of synthetic intermediate. Base-mediated reaction of the methanesulfonyl carbonates with alcohols, thiols, and amines yields carbonates, thiocarbonates, and carbamates, respectively. Overall
表征谱图
-
氢谱1HNMR
-
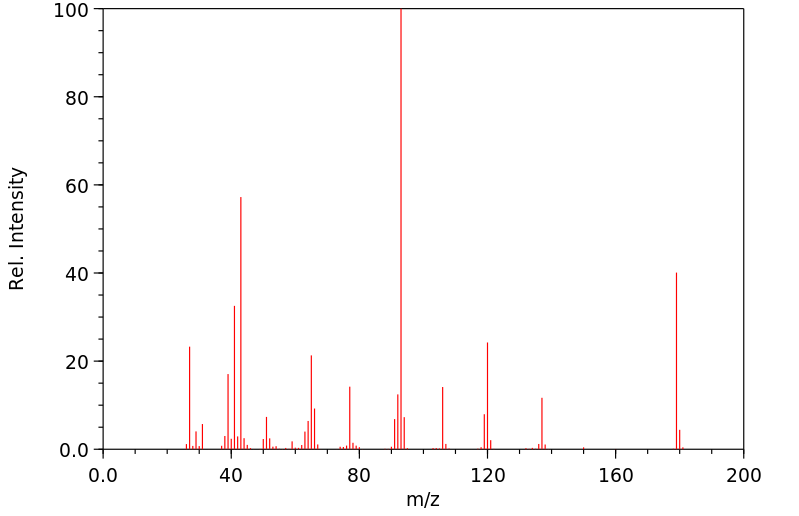
质谱MS
-
碳谱13CNMR
-
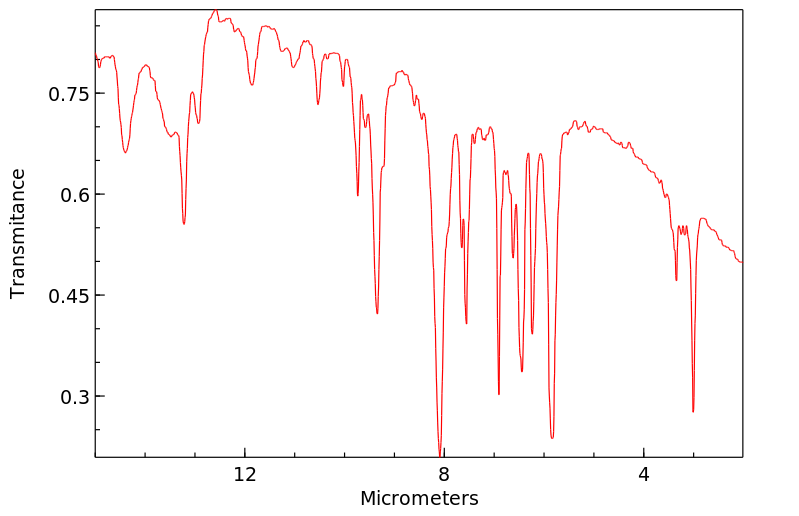
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫