DL-4-羟基扁桃酸单水化合物 | 184901-84-6
中文名称
DL-4-羟基扁桃酸单水化合物
中文别名
对羟基扁桃酸单水化合物;对羟基扁桃酸(单水化合物);4-羟基扁桃酸一水化合物;4-羟基扁桃酸单水化合物;对羟基扁桃酸一水化合物;DL-4-羟基扁桃酸一单水化合物;对羟基扁桃酸
英文名称
4-hydroxymandelic acid monohydrate
英文别名
hydroxy-(4-hydroxy-phenyl)-acetic acid monohydrate;4-hydroxymandelic acid hydrate;p-hydroxymandelic acid hydrate;2-Hydroxy-2-(4-hydroxyphenyl)acetic acid hydrate;2-hydroxy-2-(4-hydroxyphenyl)acetic acid;hydrate
CAS
184901-84-6;7198-10-9
化学式
C8H8O4*H2O
mdl
——
分子量
186.164
InChiKey
ATPBHLAWGXOMOR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:82-85 °C(lit.)
-
溶解度:DMSO(少量)、甲醇(少量)、水(少量)
计算性质
-
辛醇/水分配系数(LogP):-0.31
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:78.8
-
氢给体数:4
-
氢受体数:5
安全信息
-
危险品标志:Xi
-
安全说明:S26
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2918199090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Process for preparing 4-hydroxyphenylacetic acid摘要:通过在较低的脂肪羧酸或含水的较低脂肪羧酸反应介质中还原4-羟基曼德酸的过程,可以制备4-羟基苯乙酸。当使用结晶的4-羟基曼德酸作为起始物质时,可以高产率地获得高质量的4-羟基苯乙酸。公开号:US04337355A1
-
作为产物:参考文献:名称:Process for preparing 4-hydroxyphenylacetic acid摘要:通过在较低的脂肪羧酸或含水的较低脂肪羧酸反应介质中还原4-羟基曼德酸的过程,可以制备4-羟基苯乙酸。当使用结晶的4-羟基曼德酸作为起始物质时,可以高产率地获得高质量的4-羟基苯乙酸。公开号:US04337355A1
文献信息
-
3-(2-acyloxyethoxyphenyl)benzofuran-2-ones for use as stabilizers申请人:Ciba-Geigy Corporation公开号:US05428162A1公开(公告)日:1995-06-27Novel compounds of the formula (1), ##STR1## in which R.sub.1 is hydrogen or acyl, R.sub.2 to R.sub.5 are independently hydrogen, chloro, alkyl phenylalkyl, aryl, cycloalkyl, alkoxy, alkylthio, hydroxy, amino or substituted amino, R.sub.6 is hydrogen, R.sub.7 to R.sub.10 are independently hydrogen, alkyl or alkoxy, R.sub.17 or R.sub.19 is hydrogen or alkyl, and R.sub.18 is hydrogen, alkyl, aralkyl or aryl, are described for use as stabilizers for organic materials against thermal, oxidative or light-induced degradation.
-
Oxadiazoanthracene compounds for the treatment of diabetes申请人:TransTech Pharma, LLC公开号:US09120813B2公开(公告)日:2015-09-01The present invention provides methods of use of oxadiazoanthracene derivatives of the formula (I) and pharmaceutically acceptable salts thereof, wherein A, B, C, R, R1, R2, R3, R4 and R5 are as herein described, and wherein said methods of use include uses for the treatment of disorders and diseases, such as diabetes.
-
3-(acyloxyphenyl)benzofuran-2-one stabilisers申请人:Ciba-Geigy Corporation公开号:US05488117A1公开(公告)日:1996-01-30Compounds of the formula (1) ##STR1## in which R.sub.2, R.sub.3, R.sub.4 and R.sub.5 independently of one another, are hydrogen, C.sub.1 -C.sub.25 alkyl, C.sub.7 -C.sub.9 phenylalkyl, unsubstituted or C.sub.1 -C.sub.4 alkyl-substituted phenyl, unsubstituted or C.sub.1 -C.sub.4 alkyl-substituted C.sub.5 -C.sub.8 cycloalkyl; C.sub.1 -C.sub.18 alkoxy, hydroxyl, C.sub.1 -C.sub.25 alkanoyloxy, C.sub.3 -C.sub.25 alkenoyloxy, C.sub.3 -C.sub.25 alkanoyloxy which is interrupted by oxygen, sulfur or >N--R.sub.16 ; C.sub.6 -C.sub.9 cycloalkylcarbonyloxy, benzoyloxy or C.sub.1 -C.sub.12 alkyl-substituted benzoyloxy, where R.sub.16 is hydrogen or C.sub.1 -C.sub.8 alkyl, or, furthermore, the radicals R.sub.2 and R.sub.3 or the radicals R.sub.4 and R.sub.5 together with the carbon atoms to which they are bound form a phenyl ring, R.sub.4 is additionally --(CH.sub.2).sub.n --COR.sub.11, in which n is 0, 1 or 2, R.sub.11 is hydroxyl, ##STR2## R.sub.14 and R.sub.15, independently of one another, are hydrogen or C.sub.1 -C.sub.18 alkyl, M is an r-valent metal cation and r is 1, 2 or 3, R.sub.7, R.sub.8, R.sub.9 and R.sub.10, independently of one another, are hydrogen, C.sub.1 -C.sub.4 alkyl or C.sub.1 -C.sub.4 alkoxy, with the proviso that at least one of the radicals R.sub.7, R.sub.8, R.sub.9 and R.sub.10 is hydrogen and, in the case where R.sub.3, R.sub.5, R.sub.6, R.sub.7 and R.sub.10 are hydrogen, R.sub.4 is additionally a radical of the formula (2) ##STR3## in which R.sub.2, R.sub.8 and R.sub.9 are as defined above and R.sub.1 is as defined below for m=1, and R.sub.12 and R.sub.13, independently of one another, are hydrogen, C.sub.1 -C.sub.12 alkyl or phenyl, m is 1 or 2, and, in the case where m is 1, R.sub.1 is hydrogen, C.sub.1 -C.sub.25 alkanoyl, C.sub.3 -C.sub.25 alkenoyl, C.sub.3 -C.sub.25 alkanoyl which is interrupted by oxygen, sulfur or >N--R.sub.16 ; C.sub.6 -C.sub.9 cycloalkylcarbonyl, benzoyl or C.sub.1 -C.sub.12 alkyl-substituted benzoyl, R.sub.16 is as defined above and R.sub.6 is hydrogen or a radical of the formula (3) ##STR4## in which R.sub.1, R.sub.2, R.sub.3, R.sub.4, R.sub.5, R.sub.7, R.sub.8, R.sub.9 and R.sub.10 are as defined above, and, in the case where m is 2, R.sub.1 is ##STR5## in which R.sub.17 is a direct bond, C.sub.1 -C.sub.18 alkylene, C.sub.2 -C.sub.18 alkylene which is interrupted by oxygen, sulfur or >N--R.sub.16 ; C.sub.2 -C.sub.18 alkenylene, C.sub.2 -C.sub.20 alkylidene, C.sub.7 -C.sub.20 phenylalkylidene, C.sub.5 -C.sub.8 cycloalkylene, C.sub.7 -C.sub.8 bicycloalkylene or phenylene, R.sub.16 is as defined above and R.sub.6 is hydrogen, are described as stabilizers for organic materials against oxidative, thermal or light-induced degradation.公式(1)的化合物 ##STR1## 其中R.sub.2,R.sub.3,R.sub.4和R.sub.5独立地为氢,C.sub.1-C.sub.25烷基,C.sub.7-C.sub.9苯基烷基,未取代或C.sub.1-C.sub.4烷基取代的苯基,未取代或C.sub.1-C.sub.4烷基取代的C.sub.5-C.sub.8环烷基;C.sub.1-C.sub.18烷氧基,羟基,C.sub.1-C.sub.25脂肪酰氧基,C.sub.3-C.sub.25烯酰氧基,被氧,硫或>N--R.sub.16打断的C.sub.3-C.sub.25脂肪酰氧基;C.sub.6-C.sub.9环烷基羰氧基,苯甲酰氧基或C.sub.1-C.sub.12烷基取代的苯甲酰氧基,其中R.sub.16为氢或C.sub.1-C.sub.8烷基,或者,进一步,基团R.sub.2和R.sub.3或基团R.sub.4和R.sub.5与它们结合的碳原子一起形成苯环,R.sub.4另外为--(CH.sub.2).sub.n --COR.sub.11,其中n为0,1或2,R.sub.11为羟基,##STR2##R.sub.14和R.sub.15独立地为氢或C.sub.1-C.sub.18烷基,M为r价金属阳离子,r为1,2或3,R.sub.7,R.sub.8,R.sub.9和R.sub.10独立地为氢,C.sub.1-C.sub.4烷基或C.sub.1-C.sub.4烷氧基,但至少有一个基团R.sub.7,R.sub.8,R.sub.9和R.sub.10为氢,而且,在R.sub.3,R.sub.5,R.sub.6,R.sub.7和R.sub.10为氢的情况下,R.sub.4另外为公式(2)的基团 ##STR3## 其中R.sub.2,R.sub.8和R.sub.9如上所定义,R.sub.1如下所定义,对于m=1,R.sub.12和R.sub.13独立地为氢,C.sub.1-C.sub.12烷基或苯基,m为1或2,在m为1的情况下,R.sub.1为氢,C.sub.1-C.sub.25脂肪酰基,C.sub.3-C.sub.25烯酰基,被氧,硫或>N--R.sub.16打断的C.sub.3-C.sub.25脂肪酰基;C.sub.6-C.sub.9环烷基羰基,苯基或C.sub.1-C.sub.12烷基取代的苯基,R.sub.16如上所定义,R.sub.6为氢或公式(3)的基团 ##STR4## 其中R.sub.1,R.sub.2,R.sub.3,R.sub.4,R.sub.5,R.sub.7,R.sub.8,R.sub.9和R.sub.10如上所定义,在m为2的情况下,R.sub.1为##STR5## 其中R.sub.17为直接键,C.sub.1-C.sub.18亚烷基,被氧,硫或>N--R.sub.16打断的C.sub.2-C.sub.18亚烷基;C.sub.2-C.sub.18烯基,C.sub.2-C.sub.20烷基亚甲基,C.sub.7-C.sub.20苯基烷基亚甲基,C.sub.5-C.sub.8环烷基,C.sub.7-C.sub.8双环烷基或苯基,R.sub.16如上所定义,R.sub.6为氢,被描述为有机材料的稳定剂,用于防止氧化,热或光诱导降解。
-
3-(acyloxyphenyl)benzofuran-2-one stabilizers申请人:Ciba-Geigy Corporation公开号:US05369159A1公开(公告)日:1994-11-29Compounds of the formula (1) ##STR1## in which R.sub.2, R.sub.3, R.sub.4 and R.sub.5, independently of one another, are hydrogen, C.sub.1 -C.sub.25 alkyl, C.sub.7 -C.sub.9 phenylalkyl, unsubstituted or C.sub.1 -C.sub.4 alkyl-substituted phenyl, unsubstituted or C.sub.1 -C.sub.4 -alkly-substituted C.sub.5 -C.sub.8 cycloalkyl; C.sub.1 -C.sub.18 alkoxy, hydroxyl, C.sub.1 -C.sub.25 alkanoyloxy, C.sub.3 -C.sub.25 alkenoyloxy, C.sub.3 -C.sub.25 alkanoyloxy which is interrupted by oxygen, sulfur or >N--R.sub.16 ; C.sub.6 -C.sub.9 cycloalkylcarbonyloxy, benzoyloxy or C.sub.1 -C.sub.12 alkyl-substituted benzoyloxy, where R.sub.16 is hydrogen or C.sub.1 -C.sub.8 alkyl, or, furthermore, the radicals R.sub.2 and R.sub.3 or the radicals R.sub.4 and R.sub.5 together with the carbon atoms to which they are bound form a phenyl ring, R.sub.4 is additionally --(CH.sub.2).sub.n --COR.sub.11, in which n is 0, 1 or 2, R.sub.11 is hydroxyl, ##EQU1## C.sub.1 -C.sub.18 alkoxy or ##STR2## R.sub.14 and R.sub.15, independently of one another, are hydrogen or C.sub.1 -C.sub.18 alkyl, M is an r-valent metal cation and r is 1, 2 or 3, R.sub.7, R.sub.8, R.sub.9 and R.sub.10, independently of one another, are hydrogen, C.sub.1 -C.sub.4 alkyl or C.sub.1 -C.sub.4 alkoxy, with the proviso that at least one of the radicals R.sub.7, R.sub.8, R.sub.9 and R.sub.10 is hydrogen and, in the case where R.sub.3, R.sub.5, R.sub.6, R.sub.7 and R.sub.10 are hydrogen, R.sub.4 is additionally a radical of the formula (2) ##STR3## in which R.sub.2, R.sub.8 and R.sub.9 are as defined above and R.sub.1 is as defined below for m=1, and R.sub.12 and R.sub.13, independently of one another, are hydrogen, C.sub.1 -C.sub.12 alkyl or phenyl, m is 1 or 2, and, in the case where m is 1, R.sub.1 is hydrogen, C.sub.1 -C.sub.25 alkanoyl, C.sub.3 -C.sub.25 alkenoyl, C.sub.3 -C.sub.25 alkanoyl which is interrupted by oxygen, sulfur or >N--R.sub.16 ; C.sub.6 -C.sub.9 cycloalkylcarbonyl, benzoyl or C.sub.1 -C.sub.12 alkyl-substituted benzoyl, R.sub.16 is as defined above and R.sub.6 is hydrogen or a radical of the formula (3) ##STR4## in which R.sub.1, R.sub.2, R.sub.3, R.sub.4, R.sub.5, R.sub.7, R.sub.8, R.sub.9 and R.sub.10 are as defined above, and, in the case where m is 2, R.sub.1 is ##STR5## in which R.sub.17 is a direct bond, C.sub.1 -C.sub.18 alkylene, C.sub.2 -C.sub.18 alkylene which is interrupted by oxygen, sulfur or >N--R.sub.16 ; C.sub.2 -C.sub.18 alkenylene, C.sub.2 -C.sub.20 alkylidene, C.sub.7 -C.sub.20 phenylalkylidene, C.sub.5 -C.sub.8 cycloalkylene, C.sub.7 -C.sub.8 bicycloalkylene or phenylene, R.sub.16 is as defined above and R.sub.6 is hydrogen, are described as stabilizers for organic materials against oxidative, thermal or light-induced degradation.公式(1)的化合物##STR1##其中R.sub.2,R.sub.3,R.sub.4和R.sub.5独立地是氢,C.sub.1-C.sub.25烷基,C.sub.7-C.sub.9苯基烷基,未取代或C.sub.1-C.sub.4烷基取代的苯基,未取代或C.sub.1-C.sub.4-烷基取代的C.sub.5-C.sub.8环烷基; C.sub.1-C.sub.18烷氧基,羟基,C.sub.1-C.sub.25烷酰氧基,C.sub.3-C.sub.25烯酰氧基,C.sub.3-C.sub.25被氧、硫或>N--R.sub.16中断的烷酰氧基; C.sub.6-C.sub.9环烷基羰氧基,苯甲酰氧基或C.sub.1-C.sub.12烷基取代的苯甲酰氧基,其中R.sub.16是氢或C.sub.1-C.sub.8烷基,此外,基团R.sub.2和R.sub.3或基团R.sub.4和R.sub.5与它们结合的碳原子一起形成苯环,R.sub.4另外是--(CH.sub.2).sub.n--COR.sub.11,其中n为0, 1或2,R.sub.11为羟基,##EQU1## C.sub.1-C.sub.18烷氧基或##STR2## R.sub.14和R.sub.15独立地是氢或C.sub.1-C.sub.18烷基,M是r价金属阳离子,r为1,2或3,R.sub.7,R.sub.8,R.sub.9和R.sub.10独立地是氢,C.sub.1-C.sub.4烷基或C.sub.1-C.sub.4烷氧基,但至少有一个基团R.sub.7,R.sub.8,R.sub.9和R.sub.10是氢,在R.sub.3,R.sub.5,R.sub.6,R.sub.7和R.sub.10为氢的情况下,R.sub.4另外是公式(2)的基团##STR3##其中R.sub.2,R.sub.8和R.sub.9如上定义,R.sub.1如下定义,当m=1时,R.sub.12和R.sub.13独立地是氢,C.sub.1-C.sub.12烷基或苯基,m为1或2,当m为1时,R.sub.1为氢,C.sub.1-C.sub.25烷酰基,C.sub.3-C.sub.25烯酰基,C.sub.3-C.sub.25被氧、硫或>N--R.sub.16中断的烷酰基; C.sub.6-C.sub.9环烷基羰基,苯基或C.sub.1-C.sub.12烷基取代的苯基,R.sub.16如上定义,R.sub.6为氢或公式(3)的基团##STR4##其中R.sub.1,R.sub.2,R.sub.3,R.sub.4,R.sub.5,R.sub.7,R.sub.8,R.sub.9和R.sub.10如上定义,在m为2的情况下,R.sub.1为##STR5##其中R.sub.17是直接键,C.sub.1-C.sub.18亚基,被氧、硫或>N--R.sub.16中断的C.sub.2-C.sub.18亚基; C.sub.2-C.sub.18烯亚基,C.sub.2-C.sub.20烷基亚基,C.sub.7-C.sub.20苯基烷基亚基,C.sub.5-C.sub.8环烷基亚基,C.sub.7-C.sub.8二环烷基或苯基,R.sub.16如上定义,R.sub.6为氢,被描述为有机材料的稳定剂,抵抗氧化、热或光诱导的降解。
-
MANDELIC ACID DERIVATIVES AND PREPARATION THEREOF申请人:Polisetti Dharma Rao公开号:US20100036150A1公开(公告)日:2010-02-11The invention relates to compounds, compositions, methods of using and processes for producing optically active α-hydroxy derivatives using a combination of a chemical and biochemical methods. The process comprises reacting an ester compound with a racemic compound in the presence of an enzyme to obtain compounds (compositions and methods). The compounds produced may be useful for starting materials for physiologically active materials, functional materials and the like.
表征谱图
-
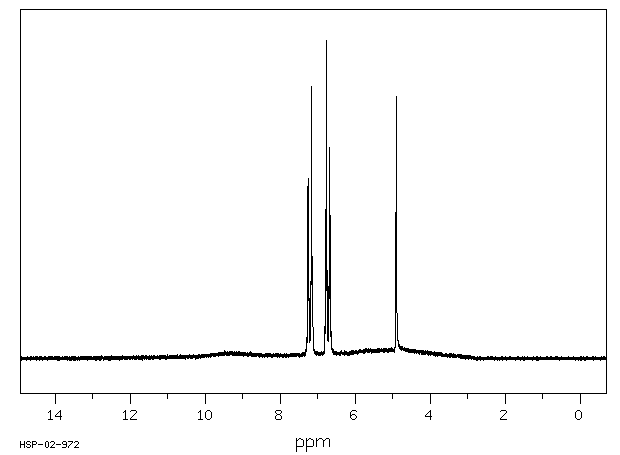
氢谱1HNMR
-
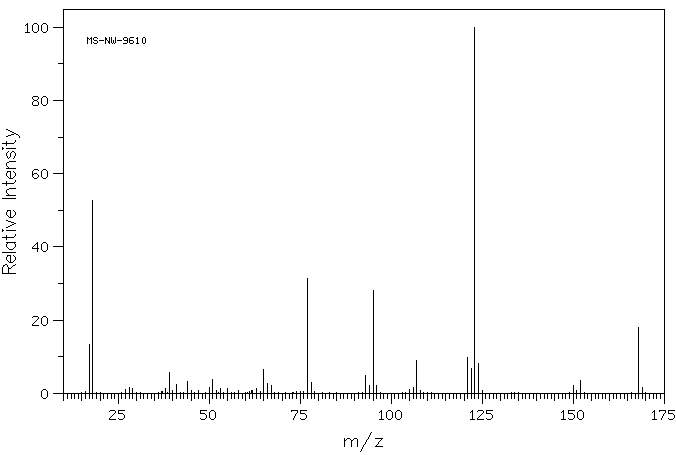
质谱MS
-
碳谱13CNMR
-
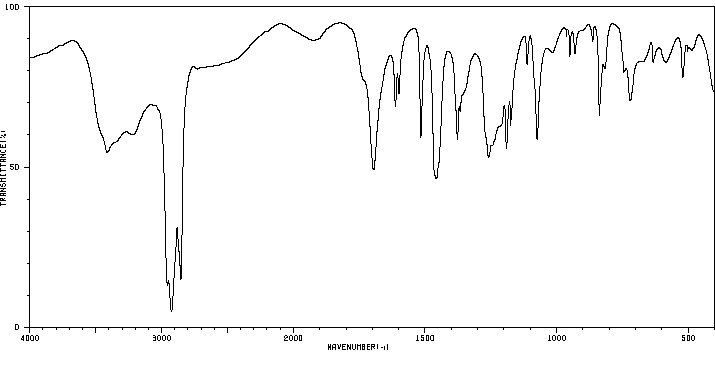
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚