苯并[f]异喹啉 | 229-67-4
中文名称
苯并[f]异喹啉
中文别名
苯并[F]异喹啉
英文名称
benzo[f]isoquinoline
英文别名
Benzisochinolin;5,6-Benzoisochinolin;Benzoisochinolin;Benz[f]isoquinoline
CAS
229-67-4
化学式
C13H9N
mdl
——
分子量
179.221
InChiKey
JQXCGCPMGZBMLE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:98-98.5 °C
-
沸点:366.0±11.0 °C(Predicted)
-
密度:1.187±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:14
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:12.9
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2933990090
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:使用 1-重氮萘醌的 Cu(II)-催化构建杂二芳基化合物:合成 QUINOX 和相关 P,N 配体的一般策略摘要:开发了一种有效且直接的方法,用于在廉价的 Cu(II) 催化下使用容易获得的N-氧化物和重氮萘醌合成杂二芳基化合物。所开发的方法以简单的方式提供了 QUINOX 和相关同类物。以高位点选择性实现了范围广泛的重要杂二芳基化合物。合成的萘酚被转化为特权相关的 P,N 配体。合适的拆分方法可以直接提供相应的轴向手性杂二芳基化合物。DOI:10.1021/acs.orglett.2c00127
-
作为产物:描述:2-(1-naphthylmethyleneamino)acetaldehyde dimethyl acetal 在 氯磺酸 作用下, 以 二氯甲烷 为溶剂, 反应 10.0h, 生成 苯并[f]异喹啉参考文献:名称:使用 1-重氮萘醌的 Cu(II)-催化构建杂二芳基化合物:合成 QUINOX 和相关 P,N 配体的一般策略摘要:开发了一种有效且直接的方法,用于在廉价的 Cu(II) 催化下使用容易获得的N-氧化物和重氮萘醌合成杂二芳基化合物。所开发的方法以简单的方式提供了 QUINOX 和相关同类物。以高位点选择性实现了范围广泛的重要杂二芳基化合物。合成的萘酚被转化为特权相关的 P,N 配体。合适的拆分方法可以直接提供相应的轴向手性杂二芳基化合物。DOI:10.1021/acs.orglett.2c00127
文献信息
-
PROCESS FOR PRODUCTION OF OXIDATION REACTION PRODUCT OF AROMATIC COMPOUND申请人:Ohkubo Kei公开号:US20120171111A1公开(公告)日:2012-07-05The present invention provides a process for producing an oxidation reaction product of an aromatic compound, having excellent environmental load reduction performance, cost reduction performance, etc. Provided is a process for producing an oxidation reaction product of a raw material aromatic compound by reacting the raw material aromatic compound with an oxidizing agent. The process further uses an electron donor-acceptor linked molecule. The process includes the step of: reacting the electron donor-acceptor linked molecule in an electron-transfer state, the oxidizing agent, and the raw material aromatic compound, thereby generating an oxidation reaction product resulting from oxidation of the raw material aromatic compound.本发明提供了一种生产芳香化合物氧化反应产物的工艺,具有优异的环境负荷减少性能、成本降低性能等。提供了一种通过将原料芳香化合物与氧化剂反应来生产原料芳香化合物的氧化反应产物的工艺。该工艺进一步使用了电子给体-受体连接分子。该工艺包括以下步骤:在电子转移状态下将电子给体-受体连接分子、氧化剂和原料芳香化合物反应,从而生成由原料芳香化合物氧化产生的氧化反应产物。
-
Enantioselective dearomatization of isoquinolines by anion-binding catalysis en route to cyclic α-aminophosphonates作者:Abhijnan Ray Choudhury、Santanu MukherjeeDOI:10.1039/c6sc02466a日期:——An enantioselective dearomatization of isoquinolines has been developed using chiral anion-binding catalysis. This transformation, catalyzed by a simple and easy to prepare tert-leucine-based thiourea derivative, makes use of silyl phosphite as a nucleophile and generates cyclic α-aminophosphonates. This is the first time asymmetric anion-binding catalysis has been applied to the synthesis of α-aminophosphonates
-
Regioselective Synthesis of Polycyclic and Heptagon-embedded Aromatic Compounds through a Versatile π-Extension of Aryl Halides作者:Wai Chung Fu、Zheng Wang、Wesley Ting Kwok Chan、Zhenyang Lin、Fuk Yee KwongDOI:10.1002/anie.201703551日期:2017.6.12halides, 2‐haloarylcarboxylic acids, and norbornadiene. The transformation is driven by the direction and subsequent decarboxylation of the carboxyl group, while norbornadiene serves as an ortho‐C−H activator and ethylene synthon via a retro‐Diels–Alder reaction. Comprehensive DFT calculations were performed to account for the catalytic intermediates.
-
A general and efficient method for the synthesis of benzo-(iso)quinoline derivatives作者:Victor Mamane、Frédéric Louërat、Julien Iehl、Mohamed Abboud、Yves FortDOI:10.1016/j.tet.2008.09.015日期:2008.11short and efficient synthesis of substituted benzo-(iso)quinoline derivatives is reported. The methodology is based on a Suzuki or Negishi cross-coupling followed by a cyclization reaction induced by t-BuOK in DMF to form the central ring. This approach allowed the synthesis of all four benzo-(iso)quinoline isomers and the substitution of each ring of the benzo-(iso)quinoline core.
-
ORGANIC COMPOUND, THREE-DIMENSIONAL ORGANIC FRAMEWORK FORMED BY USING ORGANIC COMPOUND, SEPARATION SIEVE AND OPTICAL LAYER, WHICH COMPRISE ORGANIC FRAMEWORK, AND OPTICAL DEVICE COMPRISING OPTICAL LAYER AS OPTICAL AMPLIFICATION LAYER申请人:INDUSTRY-UNIVERSITY COOPERATION FOUNDATION HANYANG UNIVERSITY公开号:US20190031586A1公开(公告)日:2019-01-31An organic compound, a three-dimensional organic structure formed by using the organic compound, a separation sieve and an optical layer having the organic structure, and an optical device having the optical layer as an optical amplification layer are provided. The organic structure includes a plurality of organic molecules self-assembled by non-covalent bonding. Each of the unit organic molecules has an aromatic ring, a first pair of substituents being connected to immediately adjacent positions of substitutable positions of the aromatic ring, and a second pair of substituents being connected to immediately adjacent positions of remaining substitutable positions of the aromatic ring. The unit organic molecules are self-assembled by van der Waals interaction, London dispersion interaction or hydrogen bonding between the first and the second pairs of the substituents and by pi-pi interactions between the aromatic rings.提供一种有机化合物,通过使用该有机化合物形成的三维有机结构、分离筛和具有该有机结构的光学层,以及具有光学层作为光学放大层的光学器件。该有机结构包括通过非共价键自组装的多个有机分子。每个单元有机分子具有芳香环,第一对取代基连接到芳香环的可取代位置的相邻位置,第二对取代基连接到芳香环的剩余可取代位置的相邻位置。单元有机分子通过范德华力相互作用、伦敦分散相互作用或氢键作用于第一和第二对取代基之间的π-π相互作用而自组装。
表征谱图
-
氢谱1HNMR
-
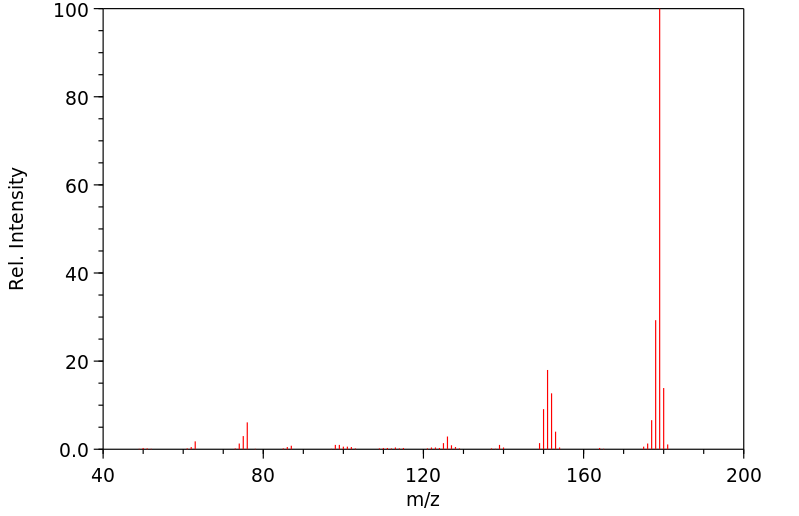
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(4-(4-氯苯基)硫代)-10-甲基-7H-benzimidazo(2,1-A)奔驰(德)isoquinolin-7一
高氯酸9-碘-11-甲基吡啶并[1,2-b]异喹啉正离子
高唐碱
顺阿曲库胺草酸盐
顺苯磺阿曲库铵叔丁酯异构体
降氧化北美黄连次碱
阿莫伦特
阿特拉库铵杂质20
阿特拉库铵杂质19
阿特拉库铵杂质19
阿曲库铵杂质V
阿曲库铵杂质N
阿曲库铵杂质J
阿曲库铵杂质I
阿曲库铵杂质F
阿曲库铵杂质E
阿曲库铵杂质E
阿曲库铵杂质D2
阿曲库铵杂质D
阿曲库铵杂质C
阿曲库铵杂质A
阿曲库铵杂质8
阿曲库铵杂质48
阿曲库铵杂质47
阿曲库铵杂质1
阿曲库铵EP杂质D
阿曲库铵
阿曲库胺草酸盐
阿司他丁
阿区库铵去甲基杂质
长茎唐松碱
过氧荧光素1
贝马力农
衡州乌药碱; 乌药碱
蝙蝠葛碱
蝙蝠葛新林碱
蒂巴因水杨酸盐
葡萄孢镰菌素
萘酞磷
萘氨磷
萘亚胺
莲心季铵碱
莲子心碱
莫沙维林
苯酚,4-[(1,2,3,4-四氢-2-甲基-1-异喹啉基)甲基]-
苯磺顺阿曲库铵杂质23
苯磺安托肌松
苯并咪唑并[2,1-A]苯并[D,E]异奎千酮-7-酮
苯并[g]异喹啉-5,10-二酮
苯并[f]异喹啉-4(3h)-酮