N-(2-羟乙基)酰丙胺 | 18266-55-2
中文名称
N-(2-羟乙基)酰丙胺
中文别名
N-丙酰乙醇胺;N-(2-羟乙基)丙酰胺
英文名称
N-(2-hydroxyethyl)propionamide
英文别名
N-(2-hydroxylethyl)propionamide;N-<2-Hydroxy-ethyl>-propionamid;N-(2-hydroxyethyl)propanamide
CAS
18266-55-2
化学式
C5H11NO2
mdl
MFCD00059600
分子量
117.148
InChiKey
GQKLTNAIFDFUDN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:46-48 °C(Solv: water (7732-18-5))
-
沸点:203 °C / 10mmHg
-
密度:1,07 g/cm3
计算性质
-
辛醇/水分配系数(LogP):-0.7
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:49.3
-
氢给体数:2
-
氢受体数:2
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2924199090
-
储存条件:存放于惰性气体中,避免与空气接触。
SDS
N-(2-Hydroxyethyl)propionamide
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: N-(2-Hydroxyethyl)propionamide
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: N-(2-Hydroxyethyl)propionamide
Percent: >97.0%(GC)
CAS Number: 18266-55-2
Synonyms: N-Propionylethanolamine
Chemical Formula: C5H11NO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Unsuitable extinguishing Solid streams of water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
N-(2-Hydroxyethyl)propionamide
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Pale yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
203°C/1.3kPa
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
Relative density: 1.07
Solubility(ies):
N-(2-Hydroxyethyl)propionamide
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
[Water] Soluble
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
N-(2-Hydroxyethyl)propionamide
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: N-(2-Hydroxyethyl)propionamide
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: N-(2-Hydroxyethyl)propionamide
Percent: >97.0%(GC)
CAS Number: 18266-55-2
Synonyms: N-Propionylethanolamine
Chemical Formula: C5H11NO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Unsuitable extinguishing Solid streams of water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
N-(2-Hydroxyethyl)propionamide
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Pale yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
203°C/1.3kPa
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
Relative density: 1.07
Solubility(ies):
N-(2-Hydroxyethyl)propionamide
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
[Water] Soluble
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
N-(2-Hydroxyethyl)propionamide
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:参考文献:名称:Process for preparing 2-oxazolines摘要:描述了一种用于制备2-噁唑啉的环脱水反应,包括在液相中反应(a)N-(2-羟基烷基)羧酰胺和(b)少量但具有催化作用的含铁化合物。该反应通常在升温条件下进行(例如180-200摄氏度),并在减压下进行。通过这种方法制备了2-H-和2-取代的2-噁唑啉。例如,通过在约180-200摄氏度/50毫米汞柱下将适当的N-(2-羟基乙基)羧酰胺与四水合氯化亚铁接触,分别制备了83.1%和90.2%产率的2-H-2-噁唑啉和2-乙基-2-噁唑啉。在这些条件下,噁唑啉产物和水基本上与它们形成的速度一样快地从反应混合物中共蒸馏出来。公开号:US04203900A1
-
作为产物:描述:参考文献:名称:一种N-乙烯基烷基酰胺的制备方法摘要:本发明涉及乙烯基化合物生产技术领域,尤其涉及一种N‑乙烯基烷基酰胺的制备方法。所述制备方法包括:A)在复合碱性催化剂的作用下,将乙醛和烷基酰胺进行反应,得到的N‑羟乙基烷基酰胺与酸酐进行酯化反应,得到酯类化合物;所述复合碱性催化剂包括无机碱和胺类化合物;B)将所述酯类化合物经中温裂解后,减压精馏得到N‑乙烯基烷基酰胺。发明人经研究发现,同时采用无机碱和胺类化合物作为催化剂,可显著降低N‑羟乙基烷基酰胺与酸酐反应所需碱性催化剂的用量,同时降低后续的裂解反应所需的温度,使反应体系更加趋于温和,在节约能耗的同时可显著提高反应产物的收率和纯度。公开号:CN112047854B
文献信息
-
Fast Monomers: Factors Affecting the Inherent Reactivity of Acrylate Monomers in Photoinitiated Acrylate Polymerization作者:Johan F. G. A. Jansen、Aylvin A. Dias、Marko Dorschu、Betty CoussensDOI:10.1021/ma021785r日期:2003.6.1study on the effect of molecular structure on the photoinitiated polymerization of acrylates was undertaken. Initially, the research was focused on the effect of hydrogen bonding, and it was found that preorganization via hydrogen bonding enhances the maximum rate of polymerization (Rp). This hydrogen bonding facilitated preorganization also affected the tacticity of the resultant polymer. Next, the
-
Synthesis and Anticonvulsant Activity of<i>N</i>-(2-Hydroxy-ethyl)amide Derivatives作者:Li-Ping Guan、Dong-Hai Zhao、Jing-Hui Xiu、Xin Sui、Hu-Ri Piao、Zhe-Shan QuanDOI:10.1002/ardp.200800153日期:2009.1N‐(2‐hydroxyethyl)amide derivatives was synthesized and screened for their anticonvulsant activities by the maximal electroshock (MES) test, and their neurotoxicity was evaluated by the rotarod test (Tox). The maximal electroshock test showed that N‐(2‐hydroxyethyl)decanamide 1g, N‐(2‐hydroxyethyl)palmitamide 1l, and N‐(2‐hydroxyeth‐yl)stearamide 1n were found to show a better anticonvulsant activity and also had合成了一系列新型 N-(2-羟乙基)酰胺衍生物,并通过最大电休克 (MES) 试验筛选其抗惊厥活性,并通过旋转棒试验 (Tox) 评估其神经毒性。最大电休克试验表明,N-(2-羟乙基)癸酰胺 1g、N-(2-羟乙基)棕榈酰胺 1l 和 N-(2-羟乙基)硬脂酰胺 1n 显示出更好的抗惊厥活性,并且具有更低的抗惊厥活性。毒性比标记的抗癫痫药丙戊酸盐要低。在抗 MES 效力测试中,这些化合物的中位有效剂量 (ED50) 分别为 22.0、23.3、20.5 mg/kg,中位毒性剂量 (TD50) 分别为 599.8、>1000、>1000 mg/kg,导致保护指数 (PI) 分别为 27.5、>42.9、>48.8。这是比标记的抗癫痫药物丙戊酸盐(PI = 1.6)更好的保护指数。为了进一步研究几种不同模型中抗惊厥活性的影响,测试了化合物 1g、1l 和 1n 引起的惊厥,这些化学物质包括戊四
-
Generation of Cyclic Ketene-<i>N</i>,<i>X</i>-Acetals (X = O, S) from 2-Alkyl-1,3-oxazolines and 2-Alkyl-1,3-thiazolines. Reactions with Acid Chlorides, 1,3-Diacid Chlorides and<i>N</i>-(Chlorocarbonyl) Isocyanate作者:Aihua Zhou、Charles U. Pittman Jr.DOI:10.1055/s-2005-918499日期:——reacted with monoacid chlorides, diacid chlorides, triacid chlorides. A series of these carbon-carbon bond-forming reactions and cyclizations to both substituted 2,3-dihydrooxazolo[3,2-a]pyridine-5,7-diones and 2,3-dihydrothiazolo[3,2-a]pyridine-5,7-diones proceeded under mild reaction conditions. Cyclic ketene-N,X-acetal intermediates play important roles in all these reactions. Related cyclizations
-
Carboxyl Activation of 2-Mercapto-4,6-dimethylpyrimidine through N-Acyl-4,6-dimethylpyrimidine-2-thione: A Chemical and Spectrophotometric Investigation作者:M.P. RajanDOI:10.14233/ajchem.2015.17607日期:——discovery of coupling reagents which gave an unprecedented impeteus to the development of peptide synthesis. Of course, in these methods high reactivity of carboxyl function towards nucleophilic attack is achieved, but rigorous conditions such as use of base, temperature etc. are required. Moreover, the electron withdrawing groups like halogen which enhances the electrophilicity of carboxyl carbon, increases与分子生物学相关的肽化学在过去的二十年里取得了巨大的进步,并且仍然是一个活跃的研究领域。最近,包括分子生物学、免疫学、药理学、酶学和神经生物学在内的生物医学研究已经确立了其重要性。此外,生物技术的进步为理解生物体的生命过程和健康科学做出了巨大贡献。这些有助于开发新的合成疫苗,可以与细菌和病毒感染、酶机制以及了解神经肽的作用竞争。使用合成肽,研究激素作用机制和酶-底物、抗原-抗体和蛋白质-DNA 相互作用的机制已经成为可能。du Vigneaud 及其同事合成了oxytoxin 后,肽的合成得到了加速。紧随其后的是混合酸酐、活性酯作为羧基活化基团的引入,以及偶联试剂的发现,这为肽合成的发展提供了前所未有的推动力。当然,在这些方法中,实现了羧基官能团对亲核攻击的高反应性,但需要严格的条件,例如使用碱、温度等。此外,像卤素这样的吸电子基团增强了羧基碳的亲电性,增加了外消旋化的可能性。现在,许多具有巯基功能的杂环系统已被证明对
-
[EN] PHENYL LINKED QUINOLINYL MODULATORS OF ROR-GAMMA-T<br/>[FR] MODULATEURS QUINOLINYLES À LIAISON PHÉNYLE DE RAR-GAMMA-T申请人:JANSSEN PHARMACEUTICA NV公开号:WO2015057203A1公开(公告)日:2015-04-23The present invention comprises compounds of Formula I. Formula I wherein: R1, R2, R3, R4, R5, R6, R7, R8, and R9 are defined in the specification. The invention also comprises a method of treating or ameliorating a syndrome, disorder or disease, wherein said syndrome, disorder or disease is rheumatoid arthritis or psoriasis. The invention also comprises a method of modulating RORγt activity in a mammal by administration of a therapeutically effective amount of at least one compound of claim 1.本发明涵盖了Formula I的化合物。其中Formula I中:R1、R2、R3、R4、R5、R6、R7、R8和R9在说明书中有定义。该发明还涵盖了一种治疗或改善综合征、疾病或疾病的方法,其中所述综合征、疾病或疾病是类风湿性关节炎或牛皮癣。该发明还涵盖了通过给予权利要求1中至少一种化合物的治疗有效量来调节哺乳动物中的RORγt活性的方法。
表征谱图
-
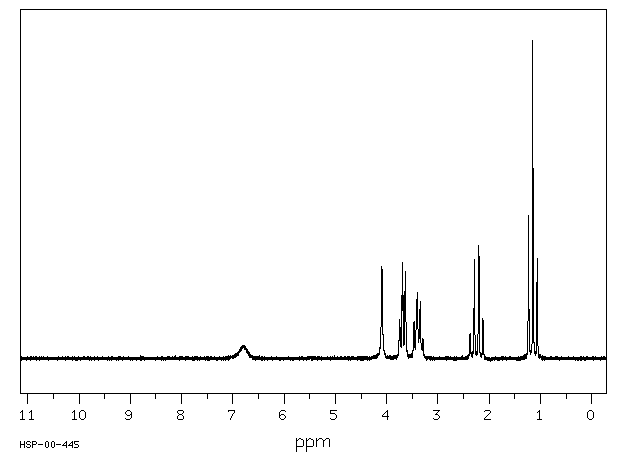
氢谱1HNMR
-
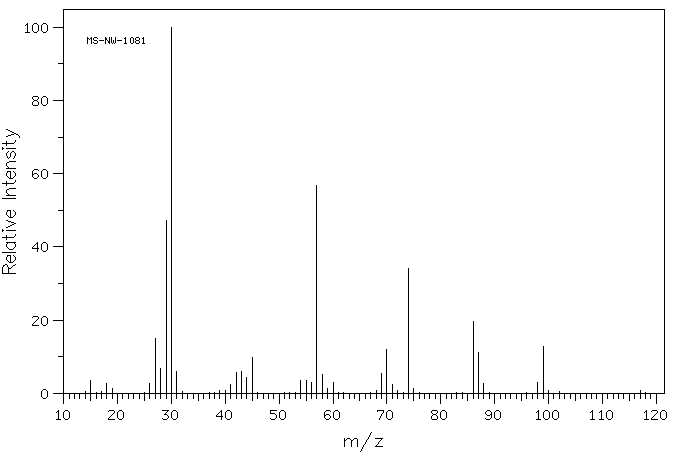
质谱MS
-
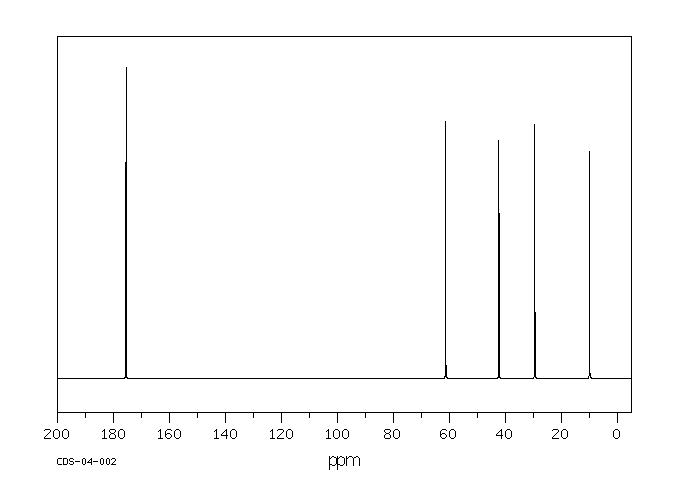
碳谱13CNMR
-
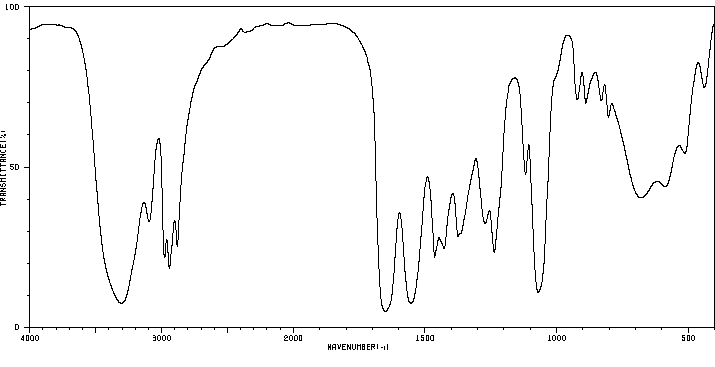
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷