2-喹喔啉硫醇 | 6962-54-5
中文名称
2-喹喔啉硫醇
中文别名
——
英文名称
quinoxaline-2-thiol
英文别名
2-mercaptoquinoxaline;1H-quinoxaline-2-thione;1H-quinoxaline-2-thione
CAS
6962-54-5
化学式
C8H6N2S
mdl
MFCD00138789
分子量
162.215
InChiKey
INQXGZFDGDSRIF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:192-195°C
-
沸点:307.0±25.0 °C(Predicted)
-
密度:1.32±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:56.5
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
安全说明:S26,S36/37/39
-
危险类别码:R36,R36/37/38
-
海关编码:2933990090
-
危险性防范说明:P261,P264,P270,P271,P280,P302+P352,P304+P340,P305+P351+P338,P312,P330,P362,P403+P233,P501
-
危险性描述:H302,H312,H332
SDS
反应信息
-
作为反应物:描述:2-喹喔啉硫醇 生成 2-(Pyridin-2-ylmethylsulfanyl)quinoxaline参考文献:名称:TAKAXASI, TOSIXIRO;XAGIVARA, KOJTIRO;NAKAMARU, KOITI;SUDZUKI, JOSIKUNI摘要:DOI:
-
作为产物:描述:参考文献:名称:S-(2,2-dihalo-1,1-difluoroethyl)-L-cysteines和S-(trihalovinyl)-L-cysteines被半胱氨酸S-共轭β-裂合酶生物激活:硫代酰基化物种和噻喃类化合物的形成迹象反应性中间体。摘要:使用19F-NMR和GC-MS分析研究了通过卤代烯烃的半胱氨酸S-共轭物的β-消除形成的反应性中间体与亲核试剂的共价结合。使用大鼠肾细胞溶质和β-裂解酶模型系统(由吡ido醛和铜(II)离子组成)进行β-消除反应。S-(1,1,2,2-四氟乙基)-L-半胱氨酸(TFE-Cys)主要转化为衍生自二氟硫代乙酰氟的产物,即二氟硫代乙酸,二氟乙酸和N-二氟硫代乙酰化的TFE-Cys。在邻苯二胺(OPD)的存在下,作为双功能亲核捕获剂,形成的主要产物是2-(二氟甲基)苯并咪唑。该产物是由二氟硫代乙酰氟与OPD的一个氨基的初始反应产生的,然后在硫代酰基和OPD的相邻氨基之间发生缩合反应。与S-(2-氯-1,1,2-三氟氟乙基)-L-半胱氨酸(CTFE-Cys)和S-(2,2-二氯-1,1-二氟氟乙基)-L-半胱氨酸(DCDFE-还通过GC-MS分析观察到硫代酰基化的半胱氨酸S-缀合物的形成,表明形成了相应的硫代酰基氟。然而,根据19DOI:10.1021/tx960049b
文献信息
-
Syntheses of sulfoxide derivatives in the benzodiazine series. Diazines. Part 37作者:Nicolas Le Fur、Ljubica Mojovic、Alain Turck、Nelly Plé、Guy Quéguiner、Vincent Reboul、Stéphane Perrio、Patrick MetznerDOI:10.1016/j.tet.2004.05.059日期:2004.8Syntheses of new sulfinylcinnolines, quinoxalines, quinazolines and phtalazines have been investigated starting from the appropriate halogenobenzodiazine derivatives. The latter were converted in one step to the corresponding sulfanyl benzodiazines which upon oxidation with m-CPBA led to the corresponding sulfoxide derivatives of benzodiazines in moderate to good yields. In parallel to this study,
-
Photocycloaddition of Quinoxaline-2(1H)-thiones to Alkenes作者:Takehiko NishioDOI:10.1002/hlca.19920750208日期:1992.3.18addition of quinoxaline-2(1H)-thiones to alkenes is described. Irradiation of the quinoxaline-2(1H)-thiones 1–4 in the presence of the alkenes 7 gave the 2-(2′-mercaptoalkyl)quinoxalines 8–11 in moderate-to-good yields via ring cleavage of an intermediate aminothietane with aromatization of the quinoxaline ring. The latter was formed by [2+2] photocycloaddition of the CS bond of the quinoxaline-2(1H)-thione
-
[EN] ANTI-NEOPLASTIC COMPOUNDS, COMPOSITIONS AND METHODS<br/>[FR] COMPOSÉS ANTINÉOPLASIQUES, COMPOSITIONS ET PROCÉDÉS
-
Quinoxalines. XXVI. Reactions of 2-quinoxalinyl thiocyanate with nucleophiles.作者:Chihoko IIJIMA、Tomiko KYODOI:10.1248/cpb.37.618日期:——2-Quinoxalinyl thiocyanate (1) possesses four electrophilic sites, i.e., 2-position, 3-position, sulfur and cyano carbon, to receive nucleophilic attack.Grignard reagents attacked preferentially the sulfur atom to give sulfides (8-12).These sulfides were readily oxidazed to sulfoxides (13-17) with sodium bromite in acetic acid.Hydroxide and ethoxide ions were found to attack preferably the cyano carbon to give thiol (2), while amines (butylamine, piperidine and morpholine) and ethyl cyanoacetate carbanion attacked the carbon at the 2-position to afford the corresponding ipso-substitution products (4-7).
-
化合物又はその薬学的に許容され得る塩、筋形成促進剤、筋萎縮抑制剤、医薬組成物及びTAZ活性化剤
表征谱图
-
氢谱1HNMR
-
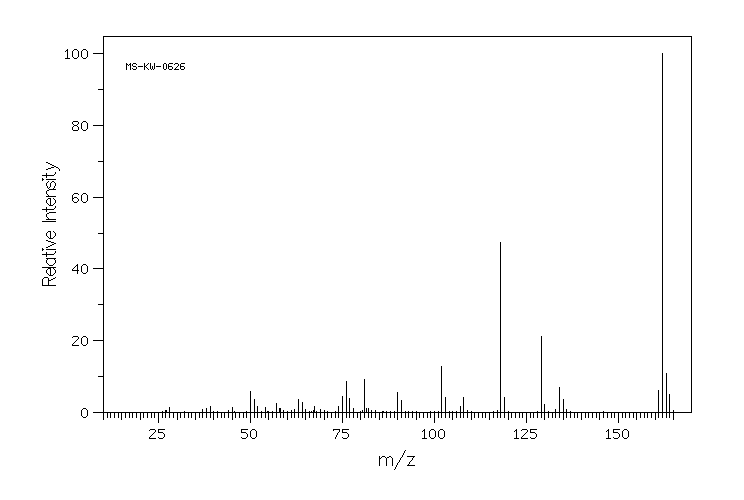
质谱MS
-
碳谱13CNMR
-
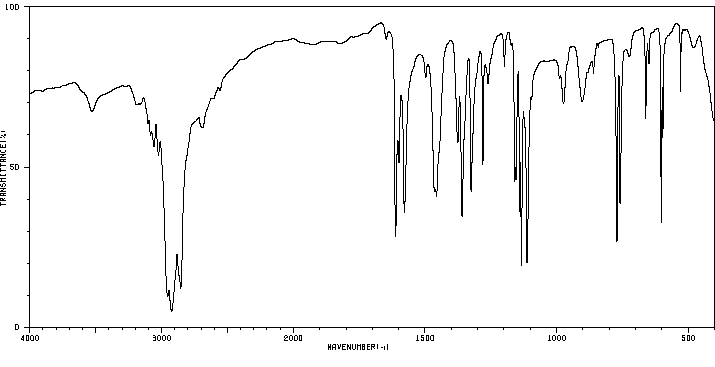
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮