(1-萘基)(2-萘基)甲烷 | 611-48-3
中文名称
(1-萘基)(2-萘基)甲烷
中文别名
——
英文名称
1,2'-Dinaphthylmethan
英文别名
1,2'-Dinaphthylmethane;1-(naphthalen-2-ylmethyl)naphthalene
CAS
611-48-3
化学式
C21H16
mdl
——
分子量
268.358
InChiKey
GPCYJQRKJVLCBS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:89-91 °C(Solv: ethyl acetate (141-78-6); hexane (110-54-3))
-
沸点:452.0±15.0 °C(Predicted)
-
密度:1.131±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):6.3
-
重原子数:21
-
可旋转键数:2
-
环数:4.0
-
sp3杂化的碳原子比例:0.05
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-萘基(2-萘基)甲醇 naphthalen-1-yl(naphthalen-2-yl)methanol 186407-93-2 C21H16O 284.357
反应信息
-
作为产物:描述:参考文献:名称:Tschitschibabin, Chemische Berichte, 1911, vol. 44, p. 442摘要:DOI:
文献信息
-
Insights into the Role of New Palladium Pincer Complexes as Robust and Recyclable Precatalysts for Suzuki-Miyaura Couplings in Neat Water作者:Blanca Inés、Raul SanMartin、María Jesús Moure、Esther DomínguezDOI:10.1002/adsc.200900345日期:2009.9Suzuki–Miyaura biaryl and diarylmethane syntheses via the coupling of arylboronic acids with aryl and arylmethyl bromides are performed in water by means of two new CNC-type palladium pincer complexes. Good to excellent results (including high TON values and extended recycling procedures) are obtained in most cases for a range of electronically dissimilar halides and boronic acids. On the basis of
-
Efficient N-heterocyclic carbene nickel pincer complexes catalyzed cross coupling of benzylic ammonium salts with boronic acids作者:Xi-Yu Liu、Hai-Bo Zhu、Ya-Jing Shen、Jian Jiang、Tao TuDOI:10.1016/j.cclet.2016.09.006日期:2017.2Abstract Pyridine-bridged bis-benzimidazolylidene nickel complexes exhibited very high catalytic activity toward cross coupling of inactive (hetero)aryl benzylic ammonium salts with (hetero)aryl and alkenyl boronic acids under mild reaction conditions. Even at 2 mol% catalyst loading, a wide range of substrates for both coupling partners with different steric and electronic properties were well tolerated
-
Nickel-Catalyzed Cross-Coupling of Anisoles with Alkyl Grignard Reagents via C–O Bond Cleavage作者:Mamoru Tobisu、Tsuyoshi Takahira、Naoto ChataniDOI:10.1021/acs.orglett.5b02200日期:2015.9.4Nickel-catalyzed cross-coupling of methoxyarenes with alkyl Grignard reagents, which involves the cleavage of the C(aryl)–OMe bond, has been developed. The use of 1,3-dicyclohexylimidazol-2-ylidene as a ligand allows the introduction of a variety of alkyl groups, including Me, Me3SiCH2, ArCH2, adamantyl, and cyclopropyl. The method can also be used for the alkylative elaboration of complex molecules
-
Gold(I)-catalyzed Benzylation of (Hetero)aryl Boronic Acids with (Hetero)benzyl Bromides by the Strategy of a S<sub>N</sub>2-type Reaction作者:Wenqing Zang、Yin Wei、Min ShiDOI:10.1002/asia.201800923日期:2018.10.4gold‐catalyzed benzylation of (hetero)aryl boronic acids with (hetero)benzyl bromides to give the corresponding cross‐coupling products in moderate to good yields is reported. The reaction proceeds through a possible intermolecular SN2‐type reaction pathway to give a wide variety of di(hetero)arylmethanes as the desired products. An intriguing reaction mechanism has been proposed on the basis of control experiments
-
Fluorous Oxime Palladacycle: A Precatalyst for Carbon–Carbon Coupling Reactions in Aqueous and Organic Medium作者:Woen Susanto、Chi-Yuan Chu、Wei Jie Ang、Tzyy-Chao Chou、Lee-Chiang Lo、Yulin LamDOI:10.1021/jo202482h日期:2012.3.16To facilitate precatalyst recovery and reuse, we have developed a fluorous, oxime-based palladacycle 1 and demonstrated that it is a very efficient and versatile precatalyst for a wide range of carbon carbon bond formation reactions (Suzuki-Miyaura, Sonogashira, Stille, Heck, Glaser-type, and Kumada) in either aqueous or organic medium under microwave irradiation. Palladacycle 1 could be recovered through F-SPE in various coupling reactions with recovery ranging from 84 to 95% for the first cycle. Inductively coupled plasma optical emission spectrometry (ICP-OES) analyses of the Pd content in the crude product from each class of transformation indicated extremely low levels of leaching and the palladacycle could be reused four to five times without significant loss of activity.
表征谱图
-
氢谱1HNMR
-
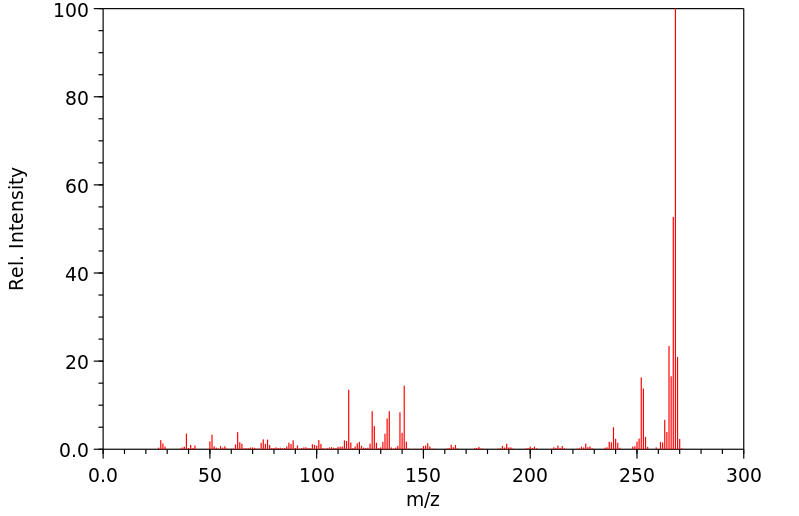
质谱MS
-
碳谱13CNMR
-
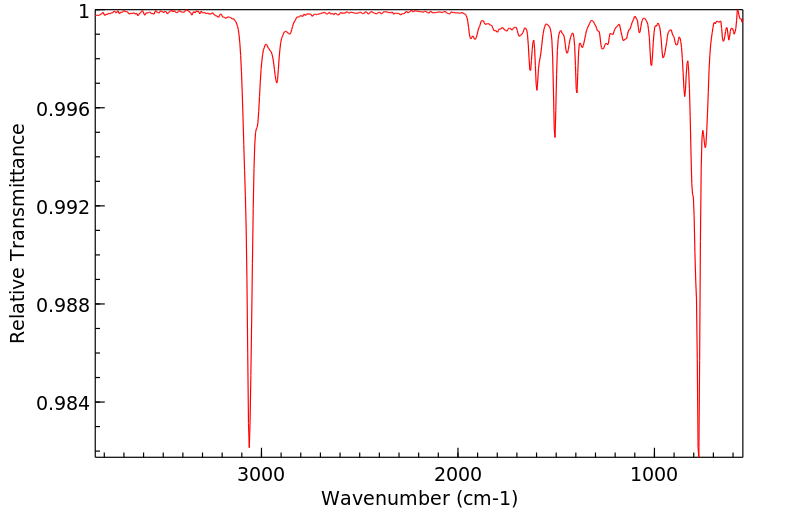
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮