2-(4-氯苯基)-3-苯基丙-2-烯腈 | 6269-09-6
中文名称
2-(4-氯苯基)-3-苯基丙-2-烯腈
中文别名
——
英文名称
(Z)-2-(4-chlorophenyl)-3-phenylacrylonitrile
英文别名
ACRYLONITRILE, 2-(p-CHLOROPHENYL)-3-PHENYL-;(Z)-2-(4-chlorophenyl)-3-phenylprop-2-enenitrile
CAS
6269-09-6
化学式
C15H10ClN
mdl
——
分子量
239.704
InChiKey
JJGLLZWUWIKTAG-GXDHUFHOSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:108-110 °C
-
沸点:369.0±30.0 °C(Predicted)
-
密度:1.211±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.3
-
重原子数:17
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:23.8
-
氢给体数:0
-
氢受体数:1
SDS
上下游信息
反应信息
-
作为反应物:描述:2-(4-氯苯基)-3-苯基丙-2-烯腈 在 dinitrogen tetraoxide 、 铁粉 、 溶剂黄146 作用下, 以 四氯化碳 为溶剂, 生成 2-(4-chloro-phenyl)-3-oxo-3-phenyl-propionic acid amide参考文献:名称:Colau,R.; Viel,C., Bulletin de la Societe Chimique de France, 1979, vol.
, p. 362 - 365 摘要:DOI: -
作为产物:描述:参考文献:名称:Reactions with 4'-Carboxy-4-Chlorostilbene摘要:DOI:10.1021/jo50007a020
文献信息
-
Pd(II)-Catalyzed C═C Bond Cleavage by a Formal Group-Exchange Reaction作者:Runyou Ye、Maoshuai Zhu、Xufei Yan、Yang Long、Ying Xia、Xiangge ZhouDOI:10.1021/acscatal.1c01850日期:2021.7.16A chelation-assisted palladium-catalyzed C═C bond cleavage of α, β-unsaturated ketone to form alkenyl nitrile in the presence of nitrile is disclosed on the basis of a formal group-exchange reaction formulated as C1═C2 + C3 → C1═C3 + C2, differing from normal alkene oxidative cleavage and metathesis type. The isolated key active Pd(II) complex as well as deuterium-labeled experiment revealed the necessity
-
Transfer Hydrogenation of Activated CC Bonds Catalyzed by Ruthenium Amido Complexes: Reaction Scope, Limitation, and Enantioselectivity作者:Dong Xue、Ying-Chun Chen、Xin Cui、Qi-Wei Wang、Jin Zhu、Jin-Gen DengDOI:10.1021/jo0478205日期:2005.4.1diamine−Ru(II)−(arene) systems was investigated to explore the asymmetric transfer hydrogenation of prochiral α,α-dicyanoolefins. Two parameters had been systematically studied, (i) the structure of the N-sulfonylated chiral diamine ligands, in which several chiral diamines substituted on the benzene ring of DPEN were first reported, and (ii) the structure of the metal precursors, and high enantioselectivitiy (up to
-
Synthesis, Characterisation and Photophysical Properties of α,β-diaryl-acrylonitrile Derivatives作者:Youfeng Yue、Haiyan Fang、Meijun Wang、Zhiyuan Wang、Mingxin YuDOI:10.3184/030823409x460687日期:2009.6α,β-Diarylacrylonitrile derivatives can be prepared by two different routes: (1) the intermolecular condensation of the same arylacetonitriles (2) the condensation of arylaldehydes and arylacetonitriles with a catalytic amount of NaOCH3 at room temperature. Several α,β-diarylacrylonitrile derivatives have been synthesised in this paper and characterised. The UV-vis absorption and photoluminescent (PL)
-
Direct C-S Bond Functionalization of Benzyl Mercaptan作者:Khokan Choudhuri、Milan Pramanik、Prasenjit MalDOI:10.1002/ejoc.202000521日期:2020.7.7Using 1,10‐phenanthroline as organocatalyst and t BuOK as base, cascaded activation of three different bonds: C(sp3)–H, benzylic C–S, and aryl–halide could be achievable in one pot.以1,10-菲咯啉为有机催化剂,以t BuOK为碱,可以在一锅中实现三个不同键的级联活化:C(sp 3)–H,苄基C–S和芳基–卤化物。
-
CuCl-Catalyzed Regio- and Stereoselective Aminohalogenation of α,β-Unsaturated Nitriles作者:Jian-Lin Han、San-Jun Zhi、Le-Yong Wang、Yi Pan、Guigen LiDOI:10.1002/ejoc.200600902日期:2007.3α,β-Unsaturated nitriles were found to be suitable substrates for aminochlorination with N,N-dichloro-p-toluenesulfonamide (4-TsNCl2) in the presence of CuCl as the catalyst (10 mol-%) and 4 A molecular sieves. The reaction is very convenient to carry out at room temperature without the protection of inert gases, and this method provides an easy route to vicinal haloamino nitriles with excellent regio-
表征谱图
-
氢谱1HNMR
-
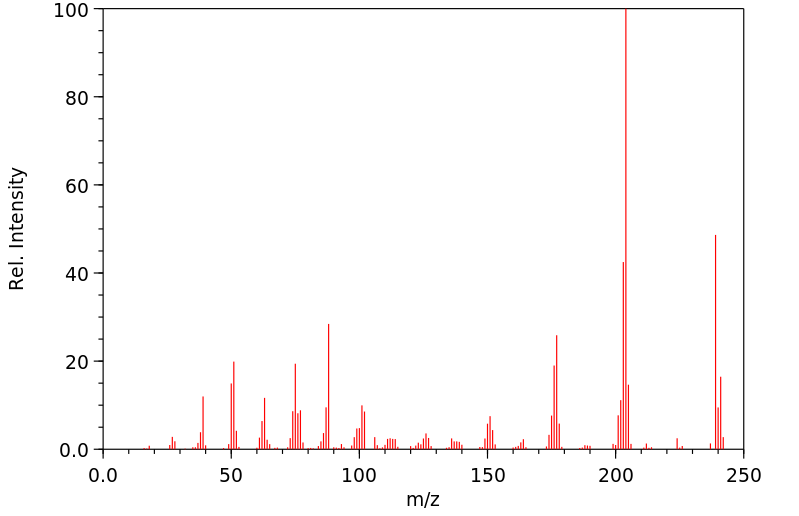
质谱MS
-
碳谱13CNMR
-
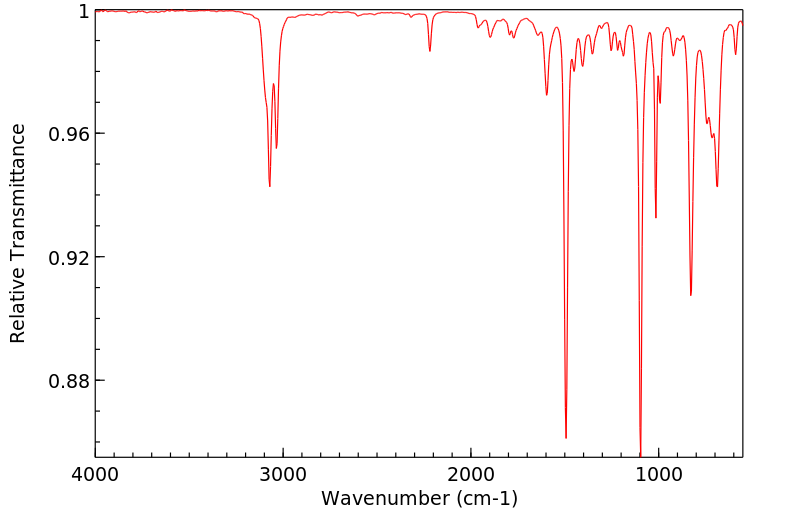
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E,Z)-他莫昔芬N-β-D-葡糖醛酸
(E/Z)-他莫昔芬-d5
(4S,5R)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S,5R,5''R)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(4R,5S)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4R,4''R,5S,5''S)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(1R,2R)-2-(二苯基膦基)-1,2-二苯基乙胺
鼓槌石斛素
黄子囊素
高黄绿酸
顺式白藜芦醇三甲醚
顺式白藜芦醇
顺式己烯雌酚
顺式-白藜芦醇3-O-beta-D-葡糖苷酸
顺式-桑皮苷A
顺式-曲札芪苷
顺式-二苯乙烯
顺式-beta-羟基他莫昔芬
顺式-a-羟基他莫昔芬
顺式-3,4',5-三甲氧基-3'-羟基二苯乙烯
顺式-1-(3-甲基-2-萘基)-2-(2-萘基)乙烯
顺式-1,2-双(三甲基硅氧基)-1,2-双(4-溴苯基)环丙烷
顺式-1,2-二苯基环丁烷
顺-均二苯乙烯硼酸二乙醇胺酯
顺-4-硝基二苯乙烯
顺-1-异丙基-2,3-二苯基氮丙啶
非洲李(PRUNUSAFRICANA)树皮提取物
阿非昔芬
阿里可拉唑
阿那曲唑二聚体
阿托伐他汀环氧四氢呋喃
阿托伐他汀环氧乙烷杂质
阿托伐他汀环(氟苯基)钠盐杂质
阿托伐他汀环(氟苯基)烯丙基酯
阿托伐他汀杂质D
阿托伐他汀杂质94
阿托伐他汀杂质7
阿托伐他汀杂质5
阿托伐他汀内酰胺钠盐杂质
阿托伐他汀中间体M4
阿奈库碘铵
锌(II)(苯甲醛)(四苯基卟啉)
银松素
铜酸盐(5-),[m-[2-[2-[1-[4-[2-[4-[[4-[[4-[2-[4-[4-[2-[2-(羧基-kO)苯基]二氮烯基-kN1]-4,5-二氢-3-甲基-5-(羰基-kO)-1H-吡唑-1-基]-2-硫代苯基]乙烯基]-3-硫代苯基]氨基]-6-(苯基氨基)-1,3,5-三嗪-2-基]氨基]-2-硫代苯基]乙烯基]-3-硫代
铒(III) 离子载体 I
铀,二(二苯基甲酮)四碘-
钾钠2,2'-[(E)-1,2-乙烯二基]二[5-({4-苯胺基-6-[(2-羟基乙基)氨基]-1,3,5-三嗪-2-基}氨基)苯磺酸酯](1:1:1)
钠{4-[氧代(苯基)乙酰基]苯基}甲烷磺酸酯
钠;[2-甲氧基-5-[2-(3,4,5-三甲氧基苯基)乙基]苯基]硫酸盐
钠4-氨基二苯乙烯-2-磺酸酯