1,2-bis[(phenylseleno)methyl]benzene | 78808-36-3
中文名称
——
中文别名
——
英文名称
1,2-bis[(phenylseleno)methyl]benzene
英文别名
1,2-bis(phenylselenomethyl)benzene;Benzene, 1,2-bis[(phenylseleno)methyl]-;1,2-bis(phenylselanylmethyl)benzene
CAS
78808-36-3
化学式
C20H18Se2
mdl
——
分子量
416.283
InChiKey
PSYSNERBXJWYAS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.75
-
重原子数:22
-
可旋转键数:6
-
环数:3.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
反应信息
-
作为反应物:描述:1,2-bis[(phenylseleno)methyl]benzene 以 乙腈 为溶剂, 以48%的产率得到5,6-二亚甲基环己-1,3-二烯参考文献:名称:通过产物分析确定短寿命中间体反应速率的双色激光光解法:在 theo-Quinodimethane 问题中的应用摘要:通过产物分析、邻醌二甲烷 (1) 和马来酸酐 (2) 环加成反应速率常数的测定,使用延时双色脉冲激光光解技术对短寿命瞬态物质进行动力学研究在室温溶液中。在过量 2 存在下,通过 KrF 准分子激光 (248 nm) 脉冲照射,从 1,2-双[(苯基硒基)甲基]苯 (3) 生成邻醌二甲烷 (1),然后连续脉冲在从 0 到 0.1 s 的不同延迟时间后,将 XeCl 准分子激光 (308 nm) 的 100 毫米辐射到反应混合物,以分解剩余的 1 以淬灭环加成反应。1 和 2 环加成的速率常数为 2.1 x 10(5) M(-1) s(-1),这是通过分析产品产量的延迟时间依赖性获得的。DOI:10.1021/ja027653v
-
作为产物:参考文献:名称:硒化物的快速热解。联苄、烯烃和相关化合物的合成摘要:研究了一系列硒化物和二硒化物的热解。硒化物和二硒化物与像苄基这样的活性亚甲基结合,以高产率得到各种取代的联苄基和相关的乙烷衍生物。其他二硒化物很容易裂解,以良好的收率与元素硒一起产生各种芳香族和脂肪族烯烃。鳞翅目、[2.2] 对环芳烷和苯并环丁烯是通过热裂解其相应的苯基硒代甲基取代化合物制备的,作为热解的应用。DOI:10.1246/bcsj.55.182
文献信息
-
<i>o</i>-Quinodimethane Generation from α,α′-Dihalo-<i>o</i>-xylenes by Use of Sodium Benzenetellurolate作者:Nobuaki Kambe、Takashi Tsukamoto、Noritaka Miyoshi、Shinji Murai、Noboru SonodaDOI:10.1246/bcsj.59.3013日期:1986.10benzenetellurolate anion to afford α,α′-bis(phenyltelluro)-o-xylene, which did not give o-quinodimethane under identical conditions. It is likely that the reaction proceeds through nucleophilic attack of benzenetellurolate anion at the tellurium atom of α-halo-α′-phenyltelluro-o-xylene which is formed in situ by the substitution of one of the halogen atoms of the starting α,α′-dihalo-o-xylene with the benzenetellurolate
-
Half-Sandwich Rhodium/Iridium(III) Complexes Designed with Cp* and 1,2-Bis(phenylchalcogenomethyl)benzene as Catalysts for Transfer Hydrogenation in Glycerol作者:Om Prakash、Kamal Nayan Sharma、Hemant Joshi、Pancham L. Gupta、Ajai K. SinghDOI:10.1021/om500149n日期:2014.5.27glycerol, which acts as a solvent and hydrogen source. Complexes 1–2 are the first examples of Rh species explored for TH in glycerol. The catalysis appears to be homogeneous. The complexes of the (Se, Se) ligand are marginally efficient than the corresponding complexes of the (S, S) ligand. The reactivity of Rh complexes in comparison to those of Ir also appears to be somewhat more. The results of DFT的反应中1,2-双(苯硫基甲基)苯(L1)和1,2-双(phenylselenomethyl)苯(L2)与[(η 5 -Cp *)的MC1(μ-Cl)的] 2(M =铑或IR)在室温下,接着用NH治疗4 PF 6已经导致空气和水分的组合物不敏感半夹心络合物[(η 5 -Cp *)M(大号)CL] [PF 6 ](RH,1 - 2; Ir,3 – 4;L = L1或L2)。他们的HR-MS,1 H,13 C 1H}和77 Se 1 H} NMR光谱被发现是特征性的。的单晶结构1 - 4已经通过X射线晶体学确定。配合物1 - 4已经发现高效的用于在甘油醛和酮的催化转移氢化(TH),其作为溶剂和氢源。复杂1 – 2是在甘油中探索TH的Rh物种的第一个实例。催化作用似乎是均相的。(Se,Se)配体的配合物比(S,S)配体的相应配合物略微有效。与Ir相比,Rh配合物的反应性似乎也更高。DFT
-
NEW SYNTHETIC METHOD OF [2.2]PARACYCLOPHANE, BENZOCYCLOBUTENE, AND LEPIDOPTERENE: PYROLYSIS OF ARYLMETHYL PHENYL SELENIDES作者:Hiroyuki Higuchi、Yoshiteru Sakata、Soichi Misumi、Tetsuo Otsubo、Fumio Ogura、Hachiro YamaguchiDOI:10.1246/cl.1981.627日期:1981.5.5The flash vacuum pyrolysis of benzyl phenyl selenides gave bibenzyls and diphenyl diselenide in excellent yields. This type of reaction was successfully applied to the synthesis of the title compounds.
-
KrF excimer laser photolysis of 1,2-bis(substituted-methyl)benzenes in the presence of alkenes and acetylene; two-photon formation of o-quinodimethane and its cycloaddition with dienophiles作者:Akihikio Ouchi、Yoshinori KogaDOI:10.1039/cc9960002075日期:——o-Quinodimethane 3 is generated effectively by Krf excimer laser (248 nm) photolysis of 1,2-bis(phenoxymethyl)-, 1,2-bis(phenylthiomethyl)- and 1,2-bis(phenylselenomthyl)-benzene via a two-photon process; cycloaddition of 3 with several dienophiles gives corresponding adducts in a maximum yield of 48%.
-
A Facile Synthesis of Tetra- and Dihydronaphthalene Derivatives by Excimer Laser Photolysis of 1,2-Bis(substituted-methyl)benzenes in the Presence of Olefins and Acetylene作者:Akihiko Ouchi、Yoshinori KogaDOI:10.1021/jo970953o日期:1997.10.1laser photolyses of 1,2-bis(phenoxymethyl)benzene (1-O), 1,2-bis[(phenylthio)methyl]benzene (1-S), and 1,2-bis[(phenylseleno)methyl]benzene (1-Se) in acetonitrile solutions via a two-photon process, which was followed by cycloaddition of 3A with several dienophiles-maleic anhydride (4a), dimethyl maleate (4b), dimethyl fumarate (4c), fumaronitrile (4d), and dimethyl acetylenedicarboxylate (4e)-to give corresponding
表征谱图
-
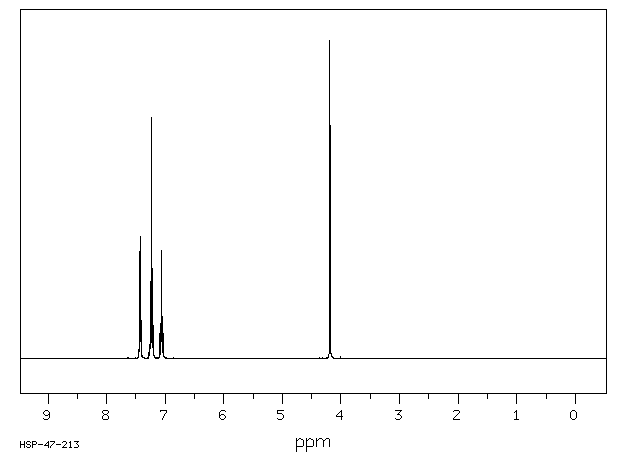
氢谱1HNMR
-
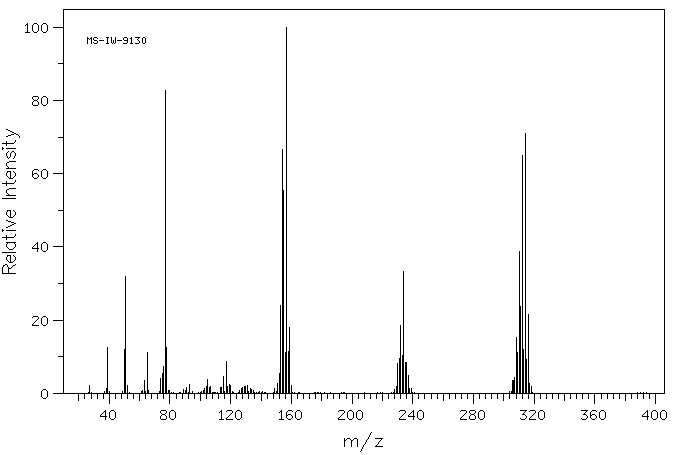
质谱MS
-
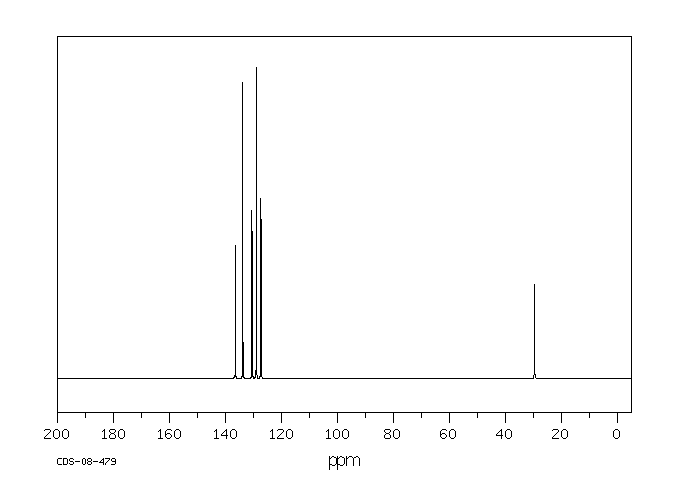
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫