1,1-二苯基-2-丙醇 | 29338-49-6
中文名称
1,1-二苯基-2-丙醇
中文别名
1,1-联苯-2-丙醇
英文名称
1,1-diphenyl-2-propanol
英文别名
1,1-diphenylpropan-2-ol;(+/-)-1.1-Diphenyl-propanol-(2)
CAS
29338-49-6
化学式
C15H16O
mdl
MFCD00004524
分子量
212.291
InChiKey
BDZAWYBXBHTHFM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:59-62 °C(lit.)
-
沸点:312.13°C (rough estimate)
-
密度:1.0018 (rough estimate)
-
稳定性/保质期:
遵照规定使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
安全说明:S24/25
-
储存条件:存于阴凉干燥处
SDS
| Name: | 1,1-Diphenyl-2-propanol, 98% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 29338-49-6 |
Synonym:
SECTION 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 29338-49-6 | 1,1-Diphenyl-2-propanol, 98% | 98 | 249-574-6 |
Risk Phrases: None Listed.
SECTION 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW The toxicological properties of this material have not been fully investigated. Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
SECTION 4 - FIRST AID MEASURES
Eyes:
Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
SECTION 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
SECTION 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
SECTION 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
SECTION 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 29338-49-6: Personal Protective Equipment
Eyes:
Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
SECTION 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystals
Color: white to off-white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 59.00 - 62.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C15H16O
Molecular Weight: 212.29
SECTION 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
SECTION 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 29338-49-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1,1-Diphenyl-2-propanol, 98% - Not listed by ACGIH, IARC, or NTP.
SECTION 12 - ECOLOGICAL INFORMATION
SECTION 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations.
SECTION 14 - TRANSPORT INFORMATION IATA Not regulated as a hazardous material. IMO Not regulated as a hazardous material. RID/ADR Not regulated as a hazardous material.
SECTION 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases: S 24/25 Avoid contact with skin and eyes. S 28A After contact with skin, wash immediately with plenty of water. S 37 Wear suitable gloves. S 45 In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible). WGK (Water Danger/Protection) CAS# 29338-49-6: No information available. Canada None of the chemicals in this product are listed on the DSL/NDSL list. CAS# 29338-49-6 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 29338-49-6 is not listed on the TSCA inventory. It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
MSDS Creation Date: 12/21/1998 Revision #2 Date: 3/18/2003 The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no way shall the company be liable for any claims, losses, or damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary damages, howsoever arising, even if the company has been advised of the possibility of such damages.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,1-二苯基丙酮 1,1-diphenylacetone 781-35-1 C15H14O 210.276 1,1-二苯基-1,2-环氧丙烷 2-Methyl-3,3-diphenyloxiran 4741-91-7 C15H14O 210.276 联苯乙醛 2,2-diphenylethanal 947-91-1 C14H12O 196.249 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (S)-1,1-diphenylpropan-2-ol —— C15H16O 212.291 R-(-)-1-二苯基-2-丙醇 (R)-1,1-diphenyl-2-propanol 52199-85-6 C15H16O 212.291 1,1-二苯基-2-丙基甲基醚 methyl 1,1-diphenyl-2-propyl ether 123525-61-1 C16H18O 226.318 —— (+/-)-acetic acid-(1-methyl-2,2-diphenyl-ethyl ester) 108974-25-0 C17H18O2 254.329 1,1-二苯基丙酮 1,1-diphenylacetone 781-35-1 C15H14O 210.276 3,3-二苯基丙酸 3,3-Diphenylpropionic acid 606-83-7 C15H14O2 226.275
反应信息
-
作为反应物:描述:参考文献:名称:有氧生成的Des-Martin高碘素类似物的氧化催化作用摘要:高价碘试剂(例如Dess-Martin高碘烷(DMP)和2-碘氧苯甲酸(IBX))是化学合成中广泛使用的氧化剂。从双氧(O 2)产生这些试剂的策略的开发将立即使O 2的使用成为可能作为多种底物氧化反应中的末端氧化剂。最近,我们通过截留醛自氧化过程中产生的反应性氧化剂,公开了I(III)试剂的有氧合成。在这项工作中,碘苯的需氧氧化与最初生成的I(III)化合物的歧化结合而生成I(V)试剂。需氧产生的I(V)试剂具有类似于DMP的底物氧化化学性质。I(V)的需氧产生的发展使I(V)中间体在有氧氧化催化中的首次应用成为可能。DOI:10.1002/anie.201804159
-
作为产物:描述:参考文献:名称:1, 1-diphenylalkan-2-ols 的分辨率摘要:为了阐明 1,1-二苯基-链烷-2-酮的不对称还原反应过程,进行了 1,1-二苯基-链烷-2-醇的外消旋拆分。描述了各种成功的切割尝试。给出了光学纯对映体的旋转值。DOI:10.1002/ardp.19733061107
文献信息
-
Chiral-at-metal iridium complex for efficient enantioselective transfer hydrogenation of ketones作者:Cheng Tian、Lei Gong、Eric MeggersDOI:10.1039/c6cc00972g日期:——A bis-cyclometalated iridium(III) complex with metal-centered chirality catalyzes the enantioselective transfer hydrogenation of ketones with high enantioselectivities at low catalyst loadings down to 0.002 mol%. Importantly, the rate of catalysis...
-
Oxidative kinetic resolution of racemic secondary alcohols in water with chiral PNNP/Ir catalyst作者:Juanni Zhang、Xiangren Yang、Han Zhou、Yanyun Li、Zhenrong Dong、Jingxing GaoDOI:10.1039/c2gc00028h日期:——Using water as solvent, the oxidative kinetic resolution of a wide range of racemic secondary alcohols with a chiral PNNP/Ir catalyst was investigated. The catalytic reaction proceeded smoothly with excellent enantioselectivity (up to 97% ee) under mild conditions, providing an environmentally benign process to achieve optically active alcohols.
-
Reactivity of ketones in homogeneous catalytic hydrogenation with cationic rhodium complexes作者:Hiroshi Fujitsu、Eiichi Matsumura、Kenjiro Takeshita、Isao MochidaDOI:10.1039/p19810002650日期:——The catalytic hydrogenation of several ketones with cationic rhodium complexes has been investigated at 30 °C with hydrogen at atmospheric pressure. The rate of the reaction depended very much on the structure of both the ketone and phosphine ligand, with triethylphosphine giving the highest activity among the ligands used. An electron-withdrawing substituent in the ketone was found to increase its
-
Visible-Light-Mediated Anti-Markovnikov Hydration of Olefins作者:Xia Hu、Guoting Zhang、Faxiang Bu、Aiwen LeiDOI:10.1021/acscatal.6b03388日期:2017.2.3avoided the need for a transition-metal catalyst, stoichiometric borane, as well as oxidant. Both terminal and internal olefins are readily accommodated in this transformation to obtain corresponding primary and secondary alcohols in good yields with single regioselectivity. This procedure can be scaled up to gram scale with a 230 turnover number based on photocatalyst.
-
Iron Catalyzed Hydroboration of Aldehydes and Ketones作者:Sem Raj Tamang、Michael FindlaterDOI:10.1021/acs.joc.7b02020日期:2017.12.1We report an operationally convenient room temperature hydroboration of aldehydes and ketones employing Fe(acac)3 as precatalyst. The hydroboration of aldehydes and ketones proceeded efficiently at room temperature to yield, after work up, 1° and 2° alcohols; chemoselective hydroboration of aldehydes over ketones is attained under these conditions. We propose a σ-bond metathesis mechanism in which
表征谱图
-
氢谱1HNMR
-
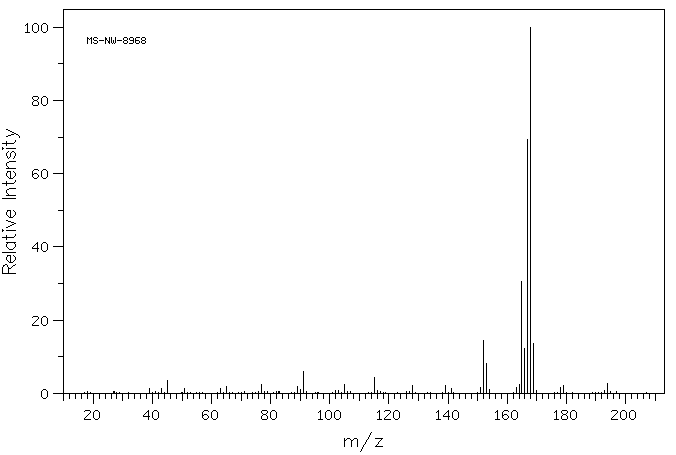
质谱MS
-
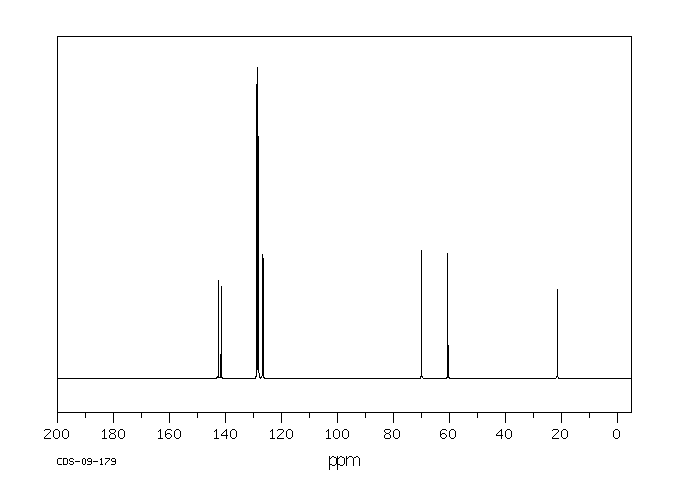
碳谱13CNMR
-
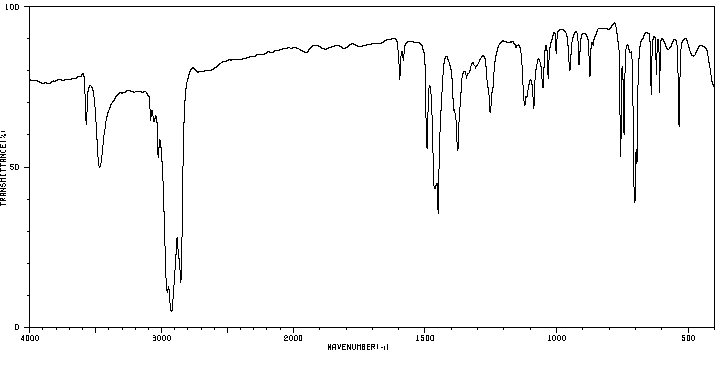
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫