2-氯-4-硫代氰酰基苯胺 | 3226-47-9
中文名称
2-氯-4-硫代氰酰基苯胺
中文别名
——
英文名称
2-chloro-4-thiocyanatoaniline
英文别名
2-Chlor-4-thiocyanato-anilin;(4-amino-3-chlorophenyl) thiocyanate
CAS
3226-47-9
化学式
C7H5ClN2S
mdl
MFCD04614735
分子量
184.649
InChiKey
TZBOJMWJGHBCEV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:75.1
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2930909090
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2-Chloro-4-thiocyanatoaniline
Synonyms: 4-Amino-3-chlorophenylthiocyanate
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Chloro-4-thiocyanatoaniline
CAS number: 3226-47-9
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H5ClN2S
Molecular weight: 184.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2-Chloro-4-thiocyanatoaniline
Synonyms: 4-Amino-3-chlorophenylthiocyanate
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Chloro-4-thiocyanatoaniline
CAS number: 3226-47-9
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H5ClN2S
Molecular weight: 184.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-chloro-4-methylthioaniline 20901-66-0 C7H8ClNS 173.666 —— 2-chloro-4-ethylthioaniline 75867-48-0 C8H10ClNS 187.693 2-氯-4-巯基苯胺 4-amino-3-chlorobenzenethiol 1122-40-3 C6H6ClNS 159.639 3-氯-4-[(氯乙酰基)氨基]硫氰酸苯酯 2-Chloro-N-(2-chloro-4-thiocyanato-phenyl)-acetamide 3082-23-3 C9H6Cl2N2OS 261.131 —— 4,4′-disulfanediylbis(2-chloroaniline) 16766-34-0 C12H10Cl2N2S2 317.263
反应信息
-
作为反应物:描述:参考文献:名称:发现 ABBV-3373,一种抗 TNF 糖皮质激素受体调节剂免疫学抗体药物偶联物摘要:使用收敛合成路线以实现多个多样性点,对一系列糖皮质激素受体调节剂(GRM)的效力、选择性和体外药物特性进行了分析。尽管涵盖了大范围的多样性,但对非缀合小分子的分析并不理想,并且使用 MP-Ala-Ala 连接子将它们与小鼠抗肿瘤坏死因子 (TNF) 抗体缀合。对所得抗体药物偶联物 (ADC) 的筛选可以更好地评估功效和物理特性,从而强化了对完整 ADC 进行结构-活性关系研究的必要性。在测量生物标志物的急性小鼠接触超敏反应模型中筛选 DAR4 ADC,以确保足够的治疗窗口。在慢性小鼠关节炎模型中,小鼠抗 TNF GRM ADC 在单剂量 10 mg/kg ip 持续 30 天以上后有效。未缀合的有效负载和小鼠替代抗 TNF ADC 的数据确定了有效负载17,该有效负载 17 与人抗 TNF 抗体缀合,并以 ABBV-3373 的形式进入临床。DOI:10.1021/acs.jmedchem.2c01579
-
作为产物:参考文献:名称:使用新型布朗斯台德酸性离子液体对芳香族和杂芳香族化合物进行区域硫代氰化反应。摘要:描述了一种方便的程序,用于制备1-(1-丙磺酸基)-3-甲基咪唑鎓硫氰酸盐作为新型的布朗斯台德酸性离子液体硫氰化剂和高效的多相催化剂。该催化剂用于在温和的H2O2和EtOH:H2O(1:1 v / v)的氧化剂存在下对吲哚,苯胺,吡咯及其衍生物(芳族和杂芳族有机化合物)进行区域选择性硫氰化。这些反应在温和且简单的条件下进行,从而以高产率和短反应时间提供区域选择性产物。DOI:10.2174/1386207319666160709191851
文献信息
-
Transition-metal-free regioselective thiocyanation of phenols, anilines and heterocycles作者:Trimbak B. Mete、Tushar M. Khopade、Ramakrishna G. BhatDOI:10.1016/j.tetlet.2016.12.043日期:2017.2An expedient direct and regioselective thiocyanation of phenols, anilines and heterocycles is described. Transformation is realized via the direct CH functionalization under transition metal free conditions at ambient temperature in excellent yields. Method proved to be monoselective and variety of functional groups tolerated the reaction conditions. The practicality of the protocol is demonstrated
-
A low-cost electrochemical thio- and selenocyanation strategy for electron-rich arenes under catalyst- and oxidant-free conditions作者:Xing Zhang、Chenguang Wang、Hong Jiang、Linhao SunDOI:10.1039/c8ra04407d日期:——
A low-cost and efficient thio- and selenocyanation strategy for electron-rich arenes has been developed under constant-current electrolytic conditions in an undivided cell without the use of any catalyst or oxidant.
-
Copper-Catalyzed Aerobic Oxidative Regioselective Thiocyanation of Aromatics and Heteroaromatics作者:Huanfeng Jiang、Wentao Yu、Xiaodong Tang、Jianxiao Li、Wanqing WuDOI:10.1021/acs.joc.7b01122日期:2017.9.15A copper-catalyzed aerobic oxidative reaction between aromatics or heteroaromatics with KSCN is developed by using O2 as the oxidant. The combination of Cu(OTf)2, N,N,N′,N′-tetramethylethylenediamine (TMEDA) and BF3·Et2O provides an efficient catalytic system, affording substituted thiocyanation products and 2-aminobenzothiazoles in excellent yields. The reaction also possesses a good functional group
-
Visible light thiocyanation of <i>N</i>-bearing aromatic and heteroaromatic compounds using Ag/TiO<sub>2</sub> nanotube photocatalyst作者:Mona Hosseini-Sarvari、Zeinab Hosseinpour、Mehdi KoohgardDOI:10.1039/c8nj03128b日期:——nanotubes (Ag/TNT) were synthesized via simple hydrothermal process, and this photocatalyst was successfully exploited in thiocyanation reactions at room temperature under visible light irradiation. Four classes of important heterocyclic compounds including indole, aniline, pyrrole and 2-amino thiazole derivatives via Ag/TNT treated with ammonium thiocyanate formed the corresponding thiocyano compounds
-
DDQ-promoted thiocyanation of aromatic and heteroaromatic compounds作者:Hamid R Memarian、Iraj Mohammadpoor-Baltork、Kobra NikoofarDOI:10.1139/v07-092日期:2007.11.1
Thiocyanation of various aromatic and heteroaromatic compounds has been achieved using ammonium thiocyanate in the presence of 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) in methanol solution at room temperature and under reflux condition. The rate of reaction is influenced by the electron-donor ability of the aromatic nucleus.Key words: amines, DDQ, indoles, thiocyanation.
表征谱图
-
氢谱1HNMR
-
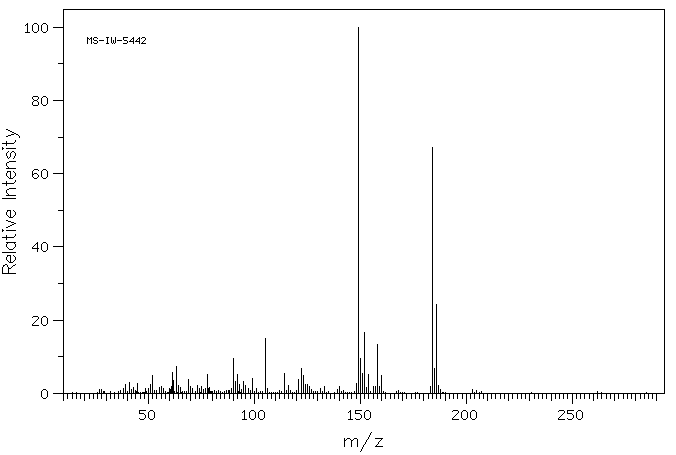
质谱MS
-
碳谱13CNMR
-
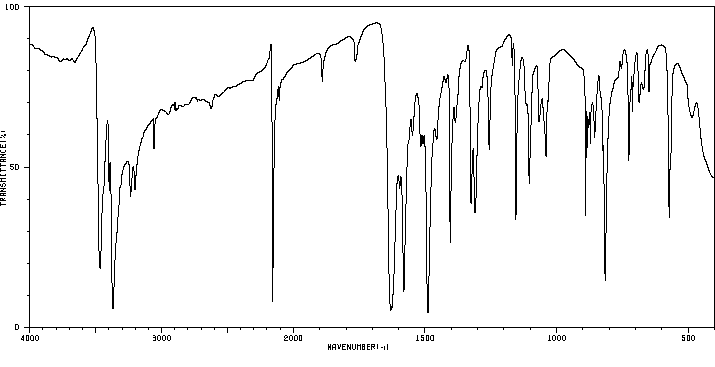
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
颜料红88
颜料紫36
顺式-1,2-二(乙硫基)-1-丙烯
非班太尔-D6
雷西那得中间体
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛-1,7-二炔-1-基(苯基)硫烷
西嗪草酮
萘,2-[(2,3-二甲基苯基)硫代]-
莫他哌那非
茴香硫醚
苯醌B
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-甲基苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2,6-二氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,2-[(2-硝基苯基)硫代]-
苯酚,3-氯-4-[(4-硝基苯基)硫代]-
苯酚,3-(乙硫基)-
苯酚,3,5-二[(苯基硫代)甲基]-
苯胺,4-[5-溴-3-[4-(甲硫基)苯基]-2-噻嗯基]-
苯胺,3-氯-4-[(1-甲基-1H-咪唑-2-基)硫代]-
苯胺,2-[(2-吡啶基甲基)硫代]-
苯硫醚-D10
苯硫胍
苯硫基乙酸
苯硫代磺酸S-(三氯乙烯基)酯
苯甲醇,2,3,4,5,6-五氟-a-[(苯基硫代)甲基]-,(R)-
苯甲酸,3-[[2-[(二甲氨基)甲基]苯基]硫代]-,盐酸
苯甲胺,5-氟-2-((3-甲氧苯基)硫代)-N,N-二甲基-,盐酸
苯甲二硫酸,4-溴苯基酯