N-2-萘基苯甲酰胺 | 18271-22-2
中文名称
N-2-萘基苯甲酰胺
中文别名
——
英文名称
N-(naphthalen-2-yl)benzamide
英文别名
N-2-naphthalenyl benzamide;N-(2-naphthyl)benzamide;N-2-Naphthylbenzamide;N-naphthalen-2-ylbenzamide
CAS
18271-22-2
化学式
C17H13NO
mdl
——
分子量
247.296
InChiKey
PBVHCWSPPOGLNA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:19
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:29.1
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2924299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-苯甲酰替苯胺 N-phenyl benzoyl amide 93-98-1 C13H11NO 197.236 2-溴-N-(萘-2-基)苯甲酰胺 2-bromo-N-naphthalen-2-ylbenzamide 571165-08-7 C17H12BrNO 326.192 —— N-hydroxy-N-(naphthalen-2-yl)benzamide —— C17H13NO2 263.296 —— [2]naphthylamino-phenyl-acetonitrile 88485-95-4 C18H14N2 258.323 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-(1-溴萘-2-基)苯甲酰胺 N-(1-bromonaphthalen-2-yl)benzamide 106184-48-9 C17H12BrNO 326.192 N-(1-硝基-2-萘基)-苯甲酰胺 N-(1-nitronaphthalen-2-yl)benzamide 6299-41-8 C17H12N2O3 292.294 —— N-Benzoyl-N-(2-naphthyl)glycine 119656-54-1 C19H15NO3 305.333
反应信息
-
作为反应物:描述:N-2-萘基苯甲酰胺 在 氧气 、 copper(II) bis(trifluoromethanesulfonate) 作用下, 以 邻二甲苯 为溶剂, 140.0 ℃ 、101.33 kPa 条件下, 反应 28.0h, 以75%的产率得到2-phenylnaphtho[2,3-d]oxazole参考文献:名称:通过铜催化苯甲酰苯胺的分子内氧化CO偶联合成2-芳基苯并恶唑。摘要:DOI:10.1002/anie.200801240
-
作为产物:描述:参考文献:名称:Petjunin et al., Zhurnal Obshchei Khimii, 1954, vol. 24, p. 178;engl.Ausg.S.181摘要:DOI:
文献信息
-
A Cross-Coupling Approach to Amide Bond Formation from Esters作者:Taoufik Ben Halima、Jaya Kishore Vandavasi、Mohanad Shkoor、Stephen G. NewmanDOI:10.1021/acscatal.7b00245日期:2017.3.3A palladium-catalyzed cross-coupling between aryl esters and anilines is reported, enabling access to diverse amides. The reaction takes place via activation of the C–O bond by oxidative addition with a Pd–NHC complex, which enables the use of relatively non-nucleophilic anilines that otherwise require stoichiometric activation with strong bases in order to react. High yields of aromatic, aliphatic
-
Efficient and Mild Ullmann-Type N-Arylation of Amides, Carbamates, and Azoles in Water作者:Maud Bollenbach、Pedro G. V. Aquino、João Xavier de Araújo-Júnior、Jean-Jacques Bourguignon、Frédéric Bihel、Christophe Salomé、Patrick Wagner、Martine SchmittDOI:10.1002/chem.201700832日期:2017.10.4A simple, sustainable, efficient, mild, and low‐cost protocol was developed for d‐glucose‐assisted Cu‐catalyzed Ullmann reactions in water for amides, carbamates, and nitrogen‐containing heterocycles. The reaction was compatible with diverse aryl/heteroaryl iodides, giving highly substituted pyridine, indole, or indazole rings. This method offers an attractive alternative to existing protocols, because
-
Solvent- and transition metal-free amide synthesis from phenyl esters and aryl amines作者:Sergey A. Rzhevskiy、Alexandra A. Ageshina、Gleb A. Chesnokov、Pavel S. Gribanov、Maxim A. Topchiy、Mikhail S. Nechaev、Andrey F. AsachenkoDOI:10.1039/c8ra10040c日期:——A general, economical, and environmentally friendly method of amide synthesis from phenyl esters and aryl amines was developed. This new method has significant advantages compared to previously reported palladium-catalyzed approaches. The reaction is performed transition metal- and solvent-free, using a cheap and environmentally benign base, NaH. This approach enabled us to obtain target amides in
-
Exploiting the Reactivity of Isocyanide: Coupling Reaction between Isocyanide and Toluene Derivatives Using the Isocyano Group as an N1 Synthon作者:Zhiqiang Liu、Xinglu Zhang、Jianxiong Li、Feng Li、Chunju Li、Xueshun Jia、Jian LiDOI:10.1021/acs.orglett.6b01928日期:2016.8.19An unusual oxidative coupling reaction of isocyanide and toluene derivatives using tetrabutylammonium iodide (TBAI) as a catalyst is disclosed. The experimental results and mechanistic study show that the isocyano group acts formally as an N1 synthon during the transformation, thus expanding the reactivity profile of isocyanide.
-
Intramolecular charge transfer with N-benzoylaminonaphthalenes. 1-Aminonaphthalene versus 2-aminonaphthalene as electron donors作者:Xuan Zhang、Chun-Hua Liu、Li-Hong Liu、Fang-Ying Wu、Lin Guo、Xiang-Ying Sun、Chao-Jie Wang、Yun-Bao JiangDOI:10.1039/b210106h日期:2003.2.11derivatives in cyclohexane and was assigned to the CT state by the observation of a substantial red shift with increasing solvent polarity or with increasing electron-withdrawing ability of the substituent. The CT emission energies were found to follow a linear relationship with the Hammett constant of the substituent and the value of the linear slope for 1-NBAs (-0.45 eV) was higher than that of 2-NBAs (-0制备了在苯甲酰基苯环的对位或间位具有不同取代基的N-(取代-苯甲酰基)-1-氨基萘和N-(取代-苯甲酰基)-2-氨基萘(1-NBA和2-NBA)使用类苯甲酰苯胺电荷转移作为探针反应,探测1-氨基萘(1-AN)和2-氨基萘(2-AN)之间的差异。对于在环己烷中所有制备的氨基萘衍生物,发现了异常的长波发射,并且通过观察到随着溶剂极性的增加或取代基的吸电子能力的增加而发生的大幅度红移,将其分配为CT状态。发现CT发射能量与取代基的Hammett常数和1-NBA(-0。45 eV高于2-NBA(-0.35 eV),后者与苯胺衍生物(BAs,-0.345 eV)接近。这表明在1-NBA的CT状态下,电荷分离的程度更高,其中完全放电分离是通过CT发射能量的线性下降率为-1.00的降低电势依赖性建立的。通过观察发现,当对位,间位和对位时,苯甲酰基取代的BA的相应线性斜率保持不变,从而排除了1-NBA和2
表征谱图
-
氢谱1HNMR
-
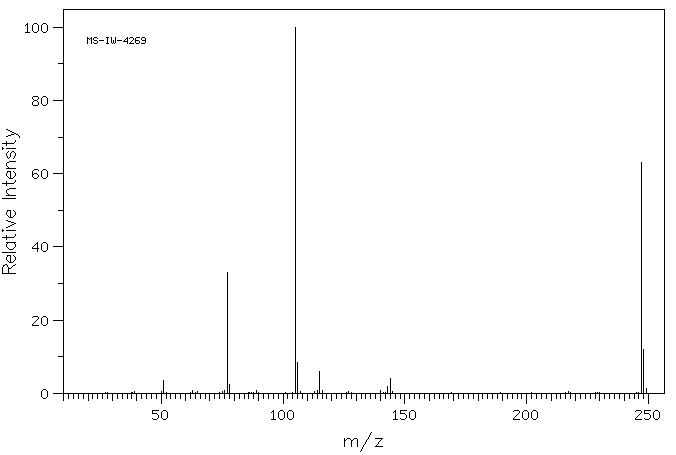
质谱MS
-
碳谱13CNMR
-
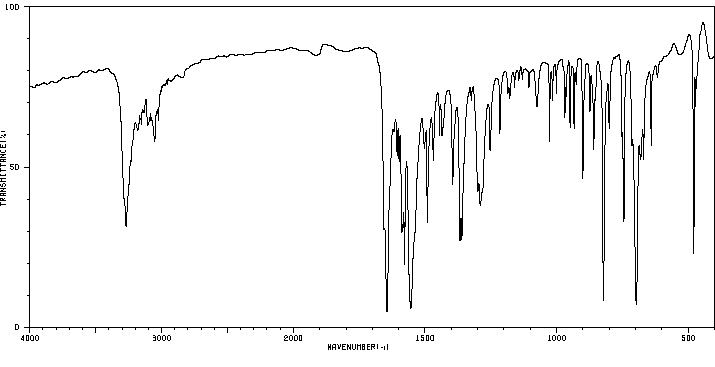
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮